Targeted ExSeq -- Tissue Preparation
Yi Cui, Anubhav Sinha, Ed Boyden, Asmamaw T. Wassie, Fei Chen, Shahar Alon
expansion microscopy
in situ sequencing
expansion sequencing
targeted ExSeq
ExSeq
spatial transcriptomics
spatial omics
spatially resolved transcriptomics
Disclaimer
This protocol is shared under the HTAN Internal Data and Materials Sharing Agreement and is provided as is. See section 14 of full agreement for full details.
This protocol is shared as an Open Access protocol (under HTAN's definitions). This protocol is Subject to IP Restrictions.
Abstract

This protocol accompanies Expansion Sequencing (ExSeq), and describes the tissue preparation for Targeted ExSeq. The steps described here are a generalization of the protocols used for figures 4-6 of the paper, and represent our recommendations for future users of the technology. Fig. 1 shows the structure of the protocol schematically.
There are three possible tissue preparation routes described in this protocol that are applicable to different experimental systems. Option (A): harvesting tissue from model organisms that can be transcardially perfused with PFA, followed by sectioning using a vibratome. We typically use this workflow for work on mouse brain sections (see figures 4-5 of ExSeq paper). Option (B): transcardially perfusing with PFA, followed by cryoprotection and cryosectioning. We occasionally use this protocol for work on mouse brain sections. Option (C): snap-freezing fresh tissue (i.e., human tumor biopsy samples, or freshly harvested tissue from mice), followed by cryoprotection and cryosectioning (see figures 2 and 6 of ExSeq paper).
The final result of options (A), (B), and (C) is the preparation of fixed tissue sections (either on a glass slide or free-floating). The protocols then briefly converge for optional antibody staining, treatment with LabelX, a chemical that enables anchoring of RNA to the expansion microscopy (ExM) hydrogel, followed by casting of the the ExM gel. There are minor differences in these steps between free-floating and slide-mounted tissue sections, which are noted in the individual steps. The next step, digestion, is tissue-type dependent and may require some optimization for your tissue type. We provide two potential options here: (1) a gentle digestion for tissues such as mouse brain, and (2) a harsh digestion for non-brain tissues such as tumor biopies. The protocols then converge again for the rest of the process.
After digestion, the gels are expanded and re-embedded within a second non-expanding hydrogel to lock in the sample size. The carboxylates within the expansion gel are then chemically passivated, enabling enzymatic reactions to be performed within the gel. The samples are now ready for library preparation.
In more detail:
Steps 1-4 describe the preparation of reagents for downstream steps. The protocol begins either along options (A)/(B), the Transcardial PFA perfusion path (Step 5, continuing to vibratome sectioning in Steps 6-7 for option (A), or cryotome sectioning in Steps 9-10 for option (B)), or along option (C), the Fresh Frozen path (Step 8, continuing to cryotome sectioning in Steps 9-10).
The protocols then converge for optional antibody staining (Step 11), followed by LabelX anchoring (Step 12), optional sample trimming (Step 13), and formation of the expansion microscopy gel (Step 14). The details of the digestion step are tissue-type dependent (Step 15). The protocol then concludes with expansion (Step 16), re-embedding (Step 17), passivation, and optional trimming (Steps 18-19).
This protocol was used to profile human metastatic breast cancer biopsies as a part of the Human Tumor Atlas Pilot Project (HTAPP). The tissue for this work was collected (see HTAPP-specific tissue collection protocol). The tissue sections were then frozen, cryosectioned, post-fixed, and permeabilized (following steps 9-10). No antibody staining was performed (skipping optional step 11). The sections were then treated with LabelX and gelled (steps 12-14). The gels were then digested using the robust digestion option in steps 15-16. The samples were then re-embedded, passivated, and trimmed (following steps 17-19).
Before start
Before starting, prepare stock solutions/reagents as described in the section Preparation of Stock Solutions (Steps 1-4).
Steps
Preparation of Stock Solutions
Common Laboratory Stocks
All solutions prepared using nuclease-free reagents, and stored at Room temperature.
- 1X PBS
- PBST (1X PBS with 0.1% Triton X-100 (v/v))
- 70% Ethanol (v/v) in water
Preparation of Reagents for LabelX RNA Anchoring
LabelX reacts with guanines of nucleic acids to install gel-anchorable moieties that can be co-polymerized with the expansion microcsopy (ExM) gel. For further technical details, see Chen*, Wassie* et al., 2016 (see Fig. 1A).
Here, we prepare the MOPS buffer (2.1) (used in the anchoring step), AcX (2.2) and LabelIT Amine (2.3) for the synthesis of LabelX, and describe the synthesis itself.
Note that the Mirus LabelIT Amine kit should be stored at -20C. The spin columns included with the kit are not used.
200 mM MOPS, pH 7.7
10X Buffer A from the Mirus LabelIT Amine kit is 200 mM MOPS, pH 7.7.
Note: only prepare additional MOPS buffer if there is not enough in the kit.
To prepare 200 mM MOPS, pH 7.7, start by dissolving the appropriate amount of MOPS powder in water.
Add small amount of 5 M NaOH solution slowly until the pH reaches pH 7.7 (periodically sampling solution by pipetting a small amount onto narrow-range pH paper strips or using a pH meter).
Buffer can be stored at Room temperature.
If preparing MOPS from powder, the suggested volume of MOPS buffer to prepare is ~10 mL.
10 mg/mL Acryloyl-X (AcX) Stock
Upon receipt, 5 mg AcX powder stock should be stored in a parafilm-sealed 50 mL Falcon tube containing Drierite at -20°C.
To prepare 10 mg/mL AcX stock, remove from freezer and allow to come to room temperature to avoid condensation. Add 500 uL anhydrous DMSO and vortex to dissolve AcX.
Aliquot into PCR strips (~20-25 μL/tube) and seal.
Store PCR strips inside a 50 mL Falcon tube containing Drierite. Seal tube with parafilm and store at -20°C.
Aliquots should be used within 1 month of preparation.
Synthesis of LabelX
Resuspend 100 μg of LabelIT Amine (1 tube) in 100 μL of Reconstitution Buffer (part of LabelIT kit), forming 1 mg/mL LabelIT Amine stock solution.
Add 10 μL 10 mg/mL AcX stock to tube, and shake vigorously at Room temperature.
Seal with parafilm and store tube at -20°C.
Preparation of Stock Solutions for Primary (Expansion) Gel
These steps describe the preparation of solutions needed for casting the ExM gel. A brief overview of the gelation process is provided in step 14.
There are four gelling reagents prepared here: (1) the monomer mix, referred to as StockX; (2) 0.5% 4-Hydroxy-TEMPO (4HT), a free radical scavenger; (3) 10% tetramethylethylenediamine (TEMED), the free radical polymerization accelerator; (4) 10% ammonium persulfate (APS), the free radical polymerization initiator.
The preparation of the 10X digestion buffer used for Proteinase-K digestion is also described here.
StockX: Gelation Monomer Solution
Prepare the following three stock solutions:
-
38% (w/v) Sodium Acrylate
-
50% (w/v) Acrylamide
-
2% (w/v) N,N'-Methylenebisacrylamide ("Bis")
Stock solutions should be sealed with parafilm and stored at -20°C.
Mix stock solutions as in table below to prepare StockX.
| A | B | C | D |
|---|---|---|---|
| Stock solution concentration | Amount | Final concentration | |
| Sodium acrylate | 38 g/100 mL (equivalent to 33% (w/w)) | 2.25 mL | 8.6 g/100 mL |
| Acrylamide | 50 g/100 mL | 0.5 mL | 2.5 g/100 mL |
| N,N′-methylenebisacrylamide | 2 g/100 mL | 0.75 mL | 0.15 g/100 mL |
| Sodium chloride | 5 M | 4 mL | 11.7 g/100 mL |
| PBS | 10X | 1 mL | 1X |
| Water | 0.9 mL | ||
| Total | 9.4 mL |
Divide StockX into 0.5 mL aliquots and store at -20°C for up to two months.
NOTE: 2% (w/v) Bis can be difficult to solubilize. A 1% Bis stock can be used if you double the volume of the Bis solution added and appropriately decrease the amount of water added.
NOTE: low-purity sodium acrylate may appear slightly yellow when dissolved in water. If this is the case, discard the solution and switch to a new bottle of sodium acrylate. Note that a number of sodium acrylate vendors, including MilliporeSigma, have had quality control issues since May 2019, leading to the inability to form gels in our hands. We currently recommend sodium acrylate from Santa Cruz Biotechnology.
0.5% 4HT
Prepare a 5% 4HT (w/v) stock. Store at -20°C for up to two months.
Prepare a working stock of 0.5% 4HT (w/v) from the 5% 4HT stock by diluting in water. The working stock can be stored at -20°C for up to a month.
To limit freeze-thaw cycles for 4HT (as well as for TEMED and APS), the suggested amount of working stock is ~200 μL.
10% TEMED
Prepare a 10% TEMED (w/v) solution in water. Store at -20°C for up to one month.
The suggested amount of 10% TEMED to prepare is ~200 μL.
Note: TEMED is toxic and corrosive. 100% stock should only be handled in the fume hood.
Note: we have found that a 10% TEMED (v/v) solution in water works essentially the same as 10% TEMED (w/v), and this is what we typically use.
10% APS
Prepare a 10% APS (w/v) solution in water. Store at -20°C for up to two weeks.
The suggested amount of 10% APS to prepare is ~200 μL.
Note: APS is toxic and reactive. The powder should only be handled in the fume hood.
Note: a stock 10% APS (w/v) solution can be used for up to two weeks for the primary (expansion) gelation, but for the re-embedding gels, 10% APS (w/v) must be fresly prepared.
10X Digestion Buffer
Prepare 10X Digestion Buffer by combining the following solutions:
| A | B | C | D |
|---|---|---|---|
| Reagent | Stock Solution Concentration | Amount | Final Concentration |
| Tris pH 8.0 | 1 M | 5 mL | 500 mM |
| EDTA | 0.5 M | 0.2 mL | 10 mM |
| Triton X-100 | 100% | 0.5 mL | 5% |
| Water | n/a | Add up to total volume of 10 mL | |
| Total | 10 mL |
Note that this does not include NaCl or Guanidine HCl; the appropriate salt is added at the time of preparation of the 1X buffer.
The 10X Digestion Buffer can be stored for up to two months at 4Room temperature.
Preparation of Stock Solutions for Passivation
Passivation is the chemical process performed after re-embedding in which the carboxylic acid functional groups in the expansion gel are converted to amides. A more detailed description is provided in step 18.
Three reagents are prepared here: (1) ethanolamine hydrochloride, the primary amine used in the amide-bond formation; (2) MES buffer, the acidic buffer used for EDC-NHS activation of the carboxylic acid; and (3) sodium borate buffer.
4 M Ethanolamine-HCl
Prepare 4 M Ethanolamine-HCl. This can be stored for up to one month at Room temperature.
The suggested volume to prepare is ~20 mL, which should be sufficient for 3-4 samples (i.e. whole-brain sections).
200 mM MES Buffer, pH 6.5
Prepare 200 mM MES (2-(N-morpholino)ethanesulfonic acid) buffer at pH 6.5 from 0.5 M stock.
The suggested volume to prepare is ~20 mL, which should be sufficient for 7-8 samples.
This can be stored for several months at Room temperature.
125 mM Sodium Borate Buffer, pH 8.5
Prepare 125 mM sodium borate buffer at pH 8.5 from 0.5 M stock.
The suggested volume to prepare is ~20 mL, which should be sufficient for 7-8 samples.
This can be stored for several months at Room temperature.
Tissue Fixation and Sectioning -- Option (A)/(B): PFA Perfusion
Transcardial PFA Perfusion and Fixation [Option (A)/(B)]
Options (A) and (B) begin with transcardial perfusion with PFA. Option (A) goes on to section the tissue using a vibratome, while option (B) sections the tissue using a cryotome. The alternative to transcardial PFA perfusion is flash freezing (option (C)), described in steps 8-10.
Step 5 describes the transcardial perfusion and fixation. Step 6 describes tissue sectioning using a vibratome, permeabilization in ethanol, and storage. Step 7 describes rehydration for downstream processing.
For cryosectioning of PFA-perfused tissue, proceed from Step 5.5 (after sinking in sucrose) to Step 8.1 for OCT embedding/freezing and subsequent sectioning.
Here, we describe the general process of PFA perfusion and fixation for mouse, optimized for harvesting mouse brain. If you have a protocol that works well in your hands for harvesting tissue for single-molecule FISH, we recommend that you use that protocol.
Preparation for Fixation
Store the following at 4°C for at least 1h 0m 0s before starting:
1 ampule of 16% (w/v) paraformaldehyde;
\~50 mL of nuclease-free water .
Freshly prepare 40 mL of 4% (w/v) paraformaldehyde in 1X PBS.
| A | B | C | D |
|---|---|---|---|
| Reagent | Stock Concentration | Amount | Final Concentration |
| Paraformaldehyde | 16% (w/v) | 10 mL (1 ampule) | 4% |
| PBS | 10X | 4 mL | 1X |
| Water | n/a | 26 mL | n/a |
| Total | 40 mL |
Additionally, aliquot/prepare 40 mL of 1X PBS (or 1X DPBS).
Store both prepared solutions (4% (w/v) paraformaldehyde in 1X PBS, and 1X PBS) at 4°C until starting procedure.
Transcardial PFA Perfusion
Follow a standard transcardial PFA perfusion protocol for in situ hybridization work. We typically perfuse an anesthetized mouse with ice-cold 1X PBS (5 to 10 mL, until perfused solution runs clear; 1X DPBS can also be used), then change to ice-cold 4% PFA in 1X PBS (10 to 20 mL).
Post-Fixation
Dissect out the tissue of interest and post-fix.
For mouse brain, we post-fix in 20-30 mL of 4% (w/v) paraformaldehyde in 1X PBS at 4°C . We avoid post-fixing for longer than one night to avoid additional autofluorescence.
[OPTIONAL] Quenching Fixation
After fixation, quench unreacted aldehydes in 20-30 mL of 1X PBS with 100 mM glycine at 4°C .
Washing with PBS
Wash the tissue with 40 mL of 1X PBS for 0h 30m 0s at 4°C.
For vibratome sectioning (option (A)), continue to Step 6.
For cryosectioning (option (B)), we cryoprotect mouse brains by sinking in 30% sucrose in 1X PBS at 4°C, before freezing (described in Steps 8-10). Other protocols may work better for different tissues. Because the tissue has already been perfused with PFA and post-fixed, the post-sectioning post-fixation (Step 9.2) is not necessary. Proceed to Step 8.
--- Pause Point ---
If needed, tissue can be washed again with 1X PBS, and stored for several days at 4°C.
Vibratome Sectioning and Storage [Option (A)]
Vibratome sectioning and permeabilization/storage conditions for tissue slices are described here.
Preparation
Fill the wells of a 24-well plastic plate approximately half-full with 70% ethanol.
Sectioning on Vibratome
Cut slices of desired thickness on a vibratome. Use 1X PBS or 1X DPBS to fill the sectioning chamber.
Transfer sections to wells of the 24-well plate, capturing 3-4 sections/well, and keeping adjacent sections in the same well.
For mouse brain, we typically cut 50 μm thick slices.
Tissue Storage and Permeabilization
Seal the plate with Parafilm and store at 4°C.
Slices are permeabilized after at least storage in 70% ethanol.
If slices are to be used immediately, they can be permeabilized in 70% ethanol for 1h 0m 0s at Room temperature.
We store slices in 70% ethanol at 4°C for extended periods of time (up to months).
Rehydration After Storage [Option (A)]
Slices require rehydration with 1X PBS after permeabilization.
Pre-fill Eppendorf tubes or the wells of an appropriate plate (typically 24-well or 48-well) with 1X PBS.
Transfer sections for further processing to the chosen wells/tubes. Multiple sections per well/tube is fine.
Wash the slices with 1X PBS for 0h 15m 0s at Room temperature.
We rehydrate slices immediately before use.
Continue to Step 11.
Tissue Fixation and Sectioning -- Option (B)/(C): Frozen Tissue Blocks
Preparation of Frozen Tissue Blocks [Option (B)/(C)]
There are two ways to prepare tissue for freezing: PFA perfusion and sucrose sinking (Option (B), continued from Step 5.5), or flash freezing (Option (C)). After freezing, the tissue is sectioned, post-fixed (for Option (C) only), permeabilized, and stored.
Step 8 describes the tissue collection (for fresh tissue) and flash freezing steps. Step 9 describes the cryosectioning, post-fixation, and permeabilization/storage steps. Step 10 describes two options for creating a chamber around the sample used for subsequent steps, as well as the rehydration washes. For option (C), follow all steps; for option (B), follow steps for freezing cryoprotected tissue
The section here is described without transcardial perfusion. One potential variation on this protocol would be to perfuse with 1X PBS to flush out blood before dissecting out the tissue of interest.
Preparation for OCT Embedding
Prepare a dry ice/isopentane bath.
Cover the bottom of a cryomold with OCT.
[Option (C) ONLY] Fresh Tissue Collection
Collect tissue of interest (i.e. harvest from anesthetized mouse, collect tumor biopsy, etc).
Freezing Tissue
Place tissue into prepared cryomold.
Add additional OCT around and on top of tissue, keeping note of the orientation of the tissue with respect to the cryomold.
[OPTIONAL] Allow cryomold to sit for ~5 minutes before freezing. For some tissues, this allows for better tissue integration with the OCT block during sectioning.
Lower cryomold into the dry ice/isopentane bath until completely frozen.
Store blocks at -80°C until cryosectioning. Frozen OCT blocks are stable for months.
Cryosectioning and Post-Fixation [Option (B)/(C)]
Cryosectioning, formalin post-fixation, permeabilization in ethanol, and storage are described here.
Cryosectioning
Section the tissue into slices of desired thickness on a cryotome, collecting sections on SuperFrost Plus slides.
Collect slides with sections in 4-well plates, keeping them cold at bottom of cryostat until post-fixation.
[Option (C) ONLY] Formalin Post-Fixation
Fix slices with ice-cold 10% Buffered Formalin for 0h 15m 0s (for 15-20 μm sections; longer for thicker sections) at Room temperature (in fume hood).
Approximately 4-5 mL of formalin is needed to cover each slide in the 4-well plates.
Post-fixation is ONLY required for fresh frozen tissue, not PFA-perfused, cryoprotected tissue.
PBS Washes
Wash the samples with 1X PBS for 0h 5m 0s at Room temperature (in fume hood).
Tissue Storage and Permeabilization
Briefly rinse slides with 70% ethanol and pipette away excess.
Then, cover slides with 70% ethanol for permeabilization.
If slides are needed immediately, permeabilization can be performed for 1h 0m 0s at Room temperature.
For permeabilization and long term storage, store slides at 4°C for at least , ideally in a parafilm-sealed screw-top container (i.e. 50 mL Falcon tube or Coplin (slide staining) jar). If using a 4-well plate for storage, keep plate in sealed, humidified container to minimize evaporation.
--- Pause Point ---
Slides can be stored in 70% Ethanol at 4°C (for up to months).
Chamber Construction and Rehydration After Storage [Option (B)/(C)]
Chambers for subsequent washes/incubations are formed directly on top of the slide-attached sections to reduce volumes for subsequent steps. There are two approaches to form the chamber -- using a hydrophobic (PAP) pen, or using a sticker to form a well.
Option 1: Constructing Chamber Around Tissue Using Hydrophobic Pen
Using the hydrophobic pen, draw a box around the tissue section, and go over the box several times to form a chamber that will stand up to fluid exchanges. The box should have a margin of a few millimeters around the edges of the tissue.
Allow the hydrophobic pen to dry completely before proceeding, typically 20 - 40 minutes.
Suggested volume for subsequent steps is 100-200 μL.
Option 2: Constructing Chamber Around Tissue Using Stickers
Place a chamber sticker (Bio-Rad Frameseal) around tissue section and seal.
Suggested volume for subsequent steps is 100-200 μL.
Rehydration Washes
Rehydrate tissue by washing with 1X PBS for 0h 15m 0s at Room temperature.
For subsequent steps, the container holding slides in a 4-well plate should remain humidified. As sealing a 4-well plate with parafilm is difficult, a tupperware container with moist KimWipes should be used to hold the plate.
[OPTIONAL] Antibody Staining for Single Target
Antibody Staining for Single Target
Because the passivation chemistry quenches many fluorophores, we perform antibody staining as a split process, decoupling staining from visualization. We perform antibody staining using a biotinylated secondary antibody. The antibody signal is subsequently recovered and imaged at the end of the in situ sequencing process by staining with fluorophore-labeled streptavidin (described in subsequent protocol).
The following steps assume that no blocking with BSA is performed, and so PBST is used in all steps. If blocking is needed, 1 - 2.5% BSA (w/v) in PBST can be prepared and used for the solutions for antibody staining. Note that the BSA should be RNAse-free.
PBST Wash
Wash with PBST for 0h 15m 0s at Room temperature.
Primary Antibody Staining
Stain with primary antibody in PBST at 4°C.
Optimal concentration should be determined and used for each antibody.
Primary Antibody Washes
Wash with PBST 0h 20m 0s at Room temperature. Washes may also need to be optimized for each antibody.
Secondary Biotinylated Antibody Staining
Stain with secondary antibody in PBST at 4°C .
Optimal concentration should be determined and used.
Secondary Antibody Washes
Wash with PBST 0h 20m 0s at Room temperature. Washes may also need to be optimized for each antibody.
LabelX Treatment of Slices
LabelX Treatment of Slices
LabelX is a bifunctional molecule, with a guanine-reactive moiety on one end (derived from LabelIT Amine), and an acryloyl group on the other (derived from AcX), enabling co-polymerization during the formation of the ExM gel.
LabelX treatment enables nucleic acids to be anchored to the expansion gel. For further details on the LabelX chemistry, see Chen*, Wassie* et al. (2016). LabelX also has some non-specific reactivity towards proteins, enabling antibodies from the antibody staining described in Step 11 to be anchored to the gel as well.
PBS Wash
Wash the sections with 1X PBS for 0h 20m 0s at Room temperature.
MOPS Buffer Pre-incubation
Wash the sections with 1X Buffer A (Mirus Kit), or 20 mM MOPS pH 7.7 buffer for 0h 20m 0s at Room temperature.
LabelX Treatment
Incubate the sections with 0.05 - 0.1 mg/mL LabelX (1:20 to 1:10 dilution) in 1X Buffer A (or MOPS) at 37°C. For free floating sections in an Eppendorf tube, ~200 uL is typically enough to submerge 1-2 sections. For sections on slides, ~100 uL is typically enough to fill one well.
We have tested 0.1 mg/mL LabelX in mouse brain and human tumor tissue extensively. We have also found that 0.05 mg/mL LabelX performs similarly in thin tumor tissue sections, which can help save on reagent cost.
PBS Washes
Wash the sections with 1X PBS for 0h 20m 0s at Room temperature.
[OPTIONAL] Trimming Intact Sections
Trimming Sections to Desired Size
To prevent gels from becoming difficult to handle after gelation and expansion (due to size), tissue sections can be trimmed down to regions of interest.
For free-floating sections, a scalpel with a curved blade is ideal, though a flat-edged razor blade will work.
For a section on a slide, a flat-edged razor blade can be used to cut and scrape off excess tissue.
Casting Expansion Gel
Preparation and Casting of Expansion Gel
The expansion gel is formed by free radical polymerization throughout the entire specimen volume. The sample is first pre-incubated with gelling solution, containing gel monomers (StockX), a free radical scavenger (4HT), a free radical polymerization accelerator (TEMED), and a free radical polymerization initiator (APS). The presence of 4HT and the pre-incubation temperature of 4°C prevents premature gelation, allowing the monomers to diffuse into the tissue. When the temperature is raised, gelation occurs. Note that oxygen inhibits gelation, so avoiding air bubbles and backfilling the gelation chamber to maximally separate the tissue from any air is important.
Preparation of Gelling Solution
Thaw aliquots of StockX, 0.5% 4HT (w/v), 10% TEMED (w/v), and 10% APS (w/v). Once thawed, keep solutions in a cold freezer block or on ice.
Mix StockX, 4HT, TEMED, and APS at a 47:1:1:1 ratio in an Eppendorf tube. Add 4HT, TEMED, and APS to StockX in that order, taking care to vortex between each addition, and keeping the solution in the cold freezer block.
Suggested volumes: if the samples are free-floating in an Eppendorf or 24/48-well plate, 400 μL total should be sufficient. If the samples are attached to a slide, 200 μL should be sufficient.
| A | B | C | D |
|---|---|---|---|
| Stock solution concentration (w/v) | Amount | Final concentration (w/v) | |
| Stock X solution | n/a | 188 µl | n/a |
| 4HT stock solution | 0.5% | 4 µl | 0.01% |
| TEMED stock solution | 10% | 4 µl | 0.2% |
| APS stock solution | 10% | 4 µl | 0.2% |
| Total | 200uL |
Pre-incubation of Sections with Gelling Solution
Transfer free-floating tissue sections from PBS to the gelling solution using a paintbrush. Transfer the Eppendorf tube/plate to the fridge for 0h 30m 0s at 4°C, ideally in/on a pre-chilled cold block.
For sections on slides, pipette out as much PBS as possible from the well, tilting the slide to help pull PBS into droplets. Pipette 100-150 μL gelling solution onto the tissue, forming a bulging well. Place the slides in a 4-well plate and transfer to the fridge for 0h 30m 0s at 4°C, ideally on top of a pre-chilled cold block.
Preparation of Gelation Chamber
Place two spacers, separated by ~10-15 mm on top of a glass slide.
Note: For free-floating tissue sections, the spacers can be placed while the sections are pre-incubating.
Note: For sections on slides, this step is performed at the end of preincubation, on the glass slide holding the section. The majority of the pre-incubation gelation solution should be removed at this stage, but the tissue section should remain covered with gelation solution. If an adhesive sticker was used to form a chamber, peel it off the glass slide. If a hydrophobic pen was used to form the chamber, scrape away the hydrophobic pen residue using a razor blade, taking to care to scrape away from the tissue.
Note: Spacers can be, for example, thickness #0 coverslips (100 μm), or a single pieces of Scotch tape (~60 μm thick). For 50 μm sections or thinner, either Scotch tape or thickness #0 coverslip is fine. For thicker sections, a coverslip of appropriate thickness should be used. For first time users, we recommend using thickness #0 coverslips, as the thicker gels are easier to handle.
Note: If coverslips are used, a 1 μL droplet of water can be pipetted onto the slide before placing the coverslip to promote better adherence between the coverslip and the glass.
Note: If Scotch tape is used as a spacer, the excess tape should be cut so as to leave no excess overhang off the edge of the slide.
Construction of Gelation Chamber
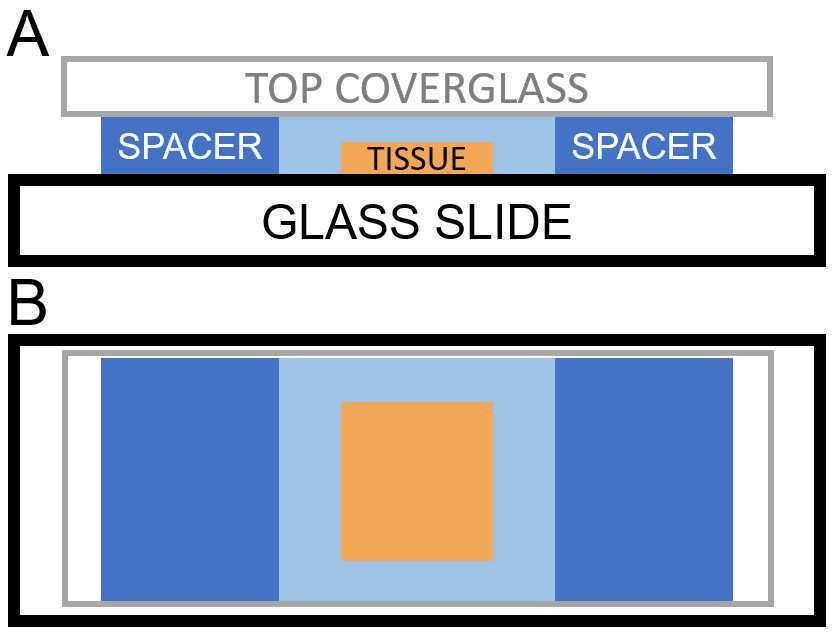
For free-floating tissue sections: When the pre-incubation is complete, pipette a droplet of gelling solution from the pre-incubation solution to the center of the chamber. Then, use a paintbrush to transfer the tissue section from the Eppendorf tube to the gelation chamber. If necessary, gently unroll/unfold the tissue with the paintbrush to flatten the tissue. Pipette an additional droplet of gelling solution on top of the gel.
For sections on slides: ensure that the tissue is covered with a droplet of gelling solution, but that there is not an excess of solution that may spill over when the lid of the gelling chamber is lowered.
Gently lower a coverslip or a slide on top of the gelling chamber, forming the lid. The lid should be lowered from one side to the other, i.e. so that the one edge of the lid is first lowered onto one of the spacers, and the second edge is slowly lowered onto the other spacer. The droplet on top of the sample will make contact with the lid and spread out over the tissue.
It is critical that bubbles near the tissue section be avoided during this process. If bubbles occur, the lid can be removed and the lowering can be attempted again.
After lowering the lid, the gelation chamber should be back-filled by pipetting additional gelation solution into the gap between the lid and the base slide. The gelling solution will spread to fill the chamber via capillary action. Fig. 2 shows the final setup of the gelation chamber.
Take care not to overfill the chamber -- the lid should not be floating. The lid coverslip should be at least thickness #2 (200 μm) to avoid bending of the coverslip, leading to a non-flat gel. If there is spillage, the excess solution can be wiped away with a KimWipe.
These steps should be completed within 5 minutes to avoid premature gelation. Eppendorf tubes containing tissue/gelling solution should be kept on a cold-block until use to minimize warming.
Gelation
Place the gelation chamber into a humidified container and incubate for 2h 0m 0s at 37°C.
Digestion and Expansion
Digestion
To enable isotropic expansion, mechanical homogenization is performed by digesting proteins in the tissue-gel composite with Proteinase-K.
The recommended digestion protocol is highly tissue-type dependent. We provide two digestion protocols here -- one for tissues that require a mild digestion to expand (i.e. mouse brain), and one for tissues that require a robust digestion to expand (most non-brain tissues, including human tumor biopsies and mouse tumor models).
For the robust digestion, a chaotropic salt (guanidine hydrochloride) is included to denature proteins and increase their accessibility for Proteinase-K digestion. In addition, increased temperature and digestion duration are used to promote increased digestion.
Preparing Digestion Buffer
Prepare the appropriate volume (described below) of either a Mild 1X Digestion Mix or a Robust 1X Digestion Mix, as is appropriate for the tissue.
Recipe for 1 mL of Mild 1X Digestion Mix (containing NaCl and Proteinase-K):
| A | B | C | D |
|---|---|---|---|
| Solution | Stock Concentration | Volume | Final Concentration |
| 10X Digestion Buffer | 10X | 100 μL | 1X |
| NaCl | 5 M | 100 μL | 500 mM |
| Proteinase-K | 800 U/mL | 10 μL | 8 U/mL |
| Water | 790 μL | ||
| Total | 1000 μL |
Recipe for 1 mL of Robust 1X Digestion Mix (containing Guanidine HCl and Proteinase-K):
| A | B | C | D |
|---|---|---|---|
| Solution | Stock Concentration | Volume | Final Concentration |
| 10X Digestion Buffer | 10X | 100 μL | 1X |
| Guanidine HCl | 8 M | 100 μL | 800 mM |
| Proteinase-K | 800 U/mL | 10 μL | 8 U/mL |
| Water | 790 μL | ||
| Total | 1000 μL |
Volume of digestion mix to prepare:
For free-floating sections: for 1-2 sections, digestion can be performed in 1 mL of 1X Digestion Mix in an Eppendorf tube. For more sections, a well of a 6-well plate is recommended, requiring 3-4 mL of 1X Digestion Mix. Additionally prepare an additional 0.5 mL of 1X Digestion Mix in a separate Eppendorf tube (Supplemental Digestion Mix). The supplemental Digestion Mix must have the appropriate salt (either NaCl or Guanidine HCl), but does not need Proteinase-K.
For sections on slides (in 4-well plates): prepare 5 mL per slide of 1X Digestion Mix.
The total volume of the Digestion Mix used should be >100 times the volume of the sample gel.
Opening Gelling Chamber
Remove the humidified container from the incubator and remove the samples.
Pry the lid off of the gelation chamber by inserting a razor blade from the side between the lid and the spacers. Take care that the gel adheres to either the lid or the base slide. If it is partly stuck to the lid and the base slide, wet a paintbrush with Supplemental Digestion Mix and gently peel the the gel off of one of the surfaces.
Make cuts between the gel and the spacers with the razor blade, then lift off the spacers in a similar fashion to the lid. Using the razor blade, trim away excess gel. Leave a small margin (~1 mm) around the tissue.
For free-floating sections: pipette ~100 μL of digestion buffer from the Supplemental Digestion Mix onto each gel. Also wet a paintbrush with Supplemental Digestion Mix. Wait 10-30 seconds for the buffer to wet the borders of the gel. Then, use the paintbrush to gently peel the gel off of the slide by slowly moving the paintbrush back and forth underneath the edge of the gel, gradually probing further underneath the gel until the entire gel lifts off. Transfer the gel to the container with 1X Digestion Mix (prepared in the previous sub-step) for digestion. If the digestion is being performed in a plate, add a few mL of water to another well to humidify the plate, then seal with parafilm.
For sections on slides: after opening the gelling chamber, place the slide (with the gel on top) into a well of a 4-well plate, and add the 1X Digestion Mix (prepared in the previous sub-step), ensuring that the gel is fully submerged in digestion mix. Add a few mL of water to another well, then place back into a humidified container for the next steps.
Option 1: Mild Digestion Conditions
Mild digestion conditions (using 1X Mild Digestion Mix) were optimized around mouse brain slices. There are three options for mild digestion that perform comparably. The lower temperature digestion conditions are preferred, as they avoid accelerating RNA degradation.
Option 1A: Digest at `Room temperature`.
Option 1B: Digest for `3h 0m 0s` at `37°C`.
Option 1C: Digest for `1h 0m 0s` at `60°C`.
The gel will expand ~1.5X during digestion. If the tissue was originally on a slide, the tissue-gel composite should lift off during digestion.
Option 2: Robust Digestion Conditions
Robust digestion conditions (using 1X Robust Digestion Mix) were optimized around tumor tissue sections on SuperFrost Plus slides.
Digest for 1h 0m 0s at 60°C.
Then, prepare a fresh batch of 1X Robust Digestion Mix, replace the digestion mix, and then incubate at 37°C.
When 24 hours from the start of digestion have elapsed, prepare a fresh batch of 1X Robust Digestion Mix, replace the digestion mix, and then incubate at 37°C again.
Digestion is complete after 48h 0m 0s of total digestion.
For sections on slides, the tissue-gel composite should have lifted off from the slide.
PBS Washes
Wash the gel with 1X PBS for 0h 15m 0s at Room temperature. The gel will be approximately 2X linearly expanded in 1X PBS.
--- Pause Point ---
The gel can be stored in 1X PBS at 4°C for up to 4 weeks. Take care to seal the container holding the gel to avoid evaporation.
Expansion
Place a glass slide at the bottom of the container that will be used for expansion. Samples will expand to 4.5X of the original size. The suggested container for expansion (and subsequent re-embedding/passivation) is a 4-well plate. A petri dish may also be used.
Transfer gel on top of the glass slide in the container.
Briefly wash the sample with water, and pipette away excess fluid.
Wash the samples with excess volumes of water for 0h 20m 0s at Room temperature.
Note: gels become fragile during the expansion process.
Casting Re-embedding Gel
Gel Re-embedding
The ExM gel changes expansion factor based on salt concentration. To lock the gel into a fixed expansion factor, a non-expanding acrylamide gel is cast within the expanded gel. This second gel embedding, called re-embedding, results in a gel that is locked at 3.3X linear expansion factor, relative to the original tissue.
Preparation of Re-embedding Solution
Prepare a fresh batch of 10% APS (as described in Step 1). Store on a cold block until use.
Prepare re-embedding solution. The volume prepared should be enough to cover the expanded gel. Suggested volume for one well of a 4-well plate is 5 mL.
Re-embedding solution:
| A | B | C | D |
|---|---|---|---|
| Solution | Stock Concentration | Volume | Final Concentration |
| Acrylamide/Bis 19:1 solution | 40% (w/v) | 375 μL | ~2.85% (w/v) acrylamide; 0.15% (w/v) N-N'-Methylenebisacrylamide |
| Water | 4525 μL | ||
| Tris Base | 1 M | 25 μL | 5 mM |
| 10% TEMED | 10% (w/v) | 37.5 μL | 0.075% |
| 10% APS | 10% (w/v) | 37.5 μL | 0.075% |
| Total | 5000 μL |
Add reagents in the listed order. Vortex after adding TEMED and APS.
Note: acrylamide is toxic and the concentrated stock should be handled in a fume hood.
Note: re-embedding solution must be used immediately.
Note: we have also used higher percentage gels (up to 4% acrylamide final concentration) without issues. Increasing the acrylamide concentration can help if you are having issues with gels breaking.
Pre-Incubation of Gels with Re-embedding Solution
Remove the final wash from the sample.
Add re-embedding solution, ensuring gels are submerged, and pre-incubate gels with very gentle shaking for 0h 30m 0s, at 25Room temperature.
Note: gel will shrink slightly (~25%) during this pre-incubation, to 3.3X linear expansion factor.
Construction of Re-embedding Chambers
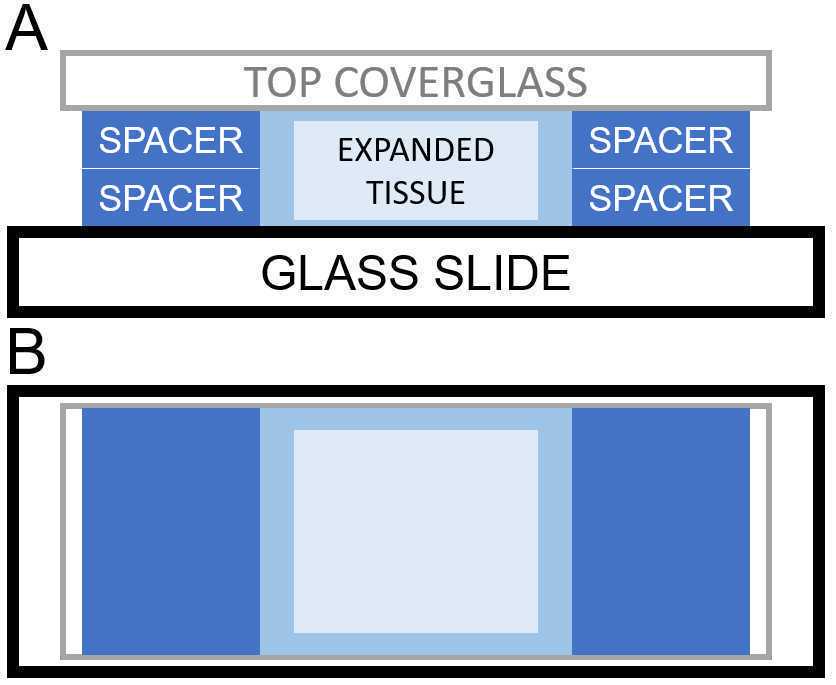
Remove samples from shaker, and remove excess re-embedding solution. Collect excess in a 50 mL Falcon tube.
Use paintbrush to gently nudge gel so that it is centered on top of the glass slide.
Place appropriate spacers on either side next to the gel. For expansion gels originally cast with scotch tape spacers, a thickness #2 (200 μm) coverslip should be sufficient. For gels originally cast with a thickness #0 coverslip spacer, two thickness #1.5 (170 μm each) coverslips stacked on top of each other should be sufficient.
To limit slippage of glass-glass contacts, small droplets (~1 μL) of water can be placed between glass surfaces to promote better adhesion.
Pipette ~20 μL of re-embedding solution on top of the gel. Then, lower the lid (a large thickness #2 coverslip, or a glass slide) on top of the chamber. As before, the lid should be lowered from one side of the gel to the other, and care should be taken to avoid trapping air bubbles.
After lowering the lid, the re-embedding chamber should be back-filled by pipetting additional re-embedding solution into the gap between the lid and the base slide. The gelling solution will spread to fill the chamber via capillary action. Take care not to overfill the chamber -- the lid should not be floating. Fig. 3 shows the setup of the final gelation chamber.
These steps should be completed within 5 minutes to avoid premature gelation.
Nitrogen Purge and Re-embedding Gelation
Place re-embedding chambers into a 4-well plate. Place the 4-well plate into a humidified Tupperware container with two small holes in the lid, and close the container.
Purge Tupperware container with nitrogen gas. To do this, start a slow flow of nitrogen gas into a nitrogen line connected to tubing with a blunt needle at the end. The flow rate should be barely perceivable when pointed at skin.
Insert the needle through one of the holes in the lid of the container. Ensure that the other hole is open, allowing for airflow out of the container. Ensure that the container does not immediately bulge or pop-open, which would be indicative of too high of a flow rate.
Purge for 0h 10m 0s.
After purging, withdraw the needle, and seal both holes by covering with tape.
Cast re-embedding by incubating for 1h 30m 0s at 37°C.
PBS Washes
Remove re-embedding chambers from incubator.
As before, use a razor blade to pry open the lid of the re-embedding chamber. Make cuts between the gel and the spacers, and remove the spacers.
Wash with 1X PBS for 0h 15m 0s at Room temperature.
If proceeding immediately to passivation, wash again with 1X PBS for 0h 45m 0s at Room temperature.
--- Pause Point ---
The re-embedding gel can be stored in a fresh wash of 1X PBS for up to 1 month at 4°C.
Passivation
Chemical Passivation of Carboxylic Acids
To enable enzymatic reactions to be performed, carboxylic acids/carboxylates within the original expansion gel need to be chemically passivated by transforming the carboxylic acid functional group into non-charged amides. This is performed by using carbodiimide crosslinker chemistry to form amides bonds between the carboxylic acids and ethanolamine. Unpassivated gels inhibit enzymatics, possibly through chelation of essential ions or interactions between the negative charges of carboxylates and enzymes.
Passivation is performed in two steps, an activation step, using EDC-NHS at pH 6.5 to activate carboxylic acids, and a coupling step at pH 8.5 to efficiently form amide bonds with ethanolamine.
Volumes here for slides in 4-well plate; can scale up/down accordingly for different containers.
Preparation of Passivation Solutions
For each sample, prepare ~3 mL of the two following solutions. Amounts for 1 mL solution are listed below.
300 mM NHS in 4M Ethanolamine hydrochloride:
| A | B | C | D |
|---|---|---|---|
| Reagent | Stock Concentration | Volume/Amount | Final Concentration |
| NHS | powder | 34.53 mg | 300 mM |
| Ethanolamine HCl solution | 4 M | 1 mL | ~4 M |
| Total | 1 mL |
300 mM EDC in 200 mM MES buffer:
| A | B | C | D |
|---|---|---|---|
| Reagent | Stock Concentration | Volume/Amount | Final Concentration |
| EDC | powder | 57.51 mg | 300 mM |
| MES Buffer | 200 mM | 1 mL | 200 mM |
| Total | 1 mL |
For both solutions, it is typically easier to mass out an approximate amount of NHS or EDC, then calculate the correct buffer volume to add to reach the correct concentration.
Passivation: Carboxylate Activation
For each sample, combine 2.5 mL of the NHS in Ethanolamine with 2.5 mL of the EDC in MES, vortex, and add to sample in 4-well plate.
Carboxylate Activation Solution:
| A | B | C |
|---|---|---|
| Solution | Volume | Final Concentration |
| 300 mM NHS in 4 M Ethanolamine HCl | 2.5 mL | 150 mM NHS; 2 M Ethanolamine |
| 300 mM EDC in 200 mM MES Buffer | 2.5 mL | 150 mM EDC; 100 mM MES |
| Total | 5 mL |
Incubate gels in 5 mL carboxylate activation solution with gentle shaking for 2h 0m 0s at Room temperature.
Passivation: Coupling
Prepare 5 mL of coupling solution.
| A | B | C | D |
|---|---|---|---|
| Solution | Stock Concentration | Volume | Final Concentration |
| Ethanolamine HCl | 4 M | 2.5 mL | 2 M |
| Sodium Borate, pH 8.5 | 125 mM | 2.5 mL | 62.5 mM |
| Total | 5 mL |
Incubate gels in 5 mL coupling solution with gentle shaking for 0h 40m 0s at Room temperature.
PBS Washes
Wash gels with 1X PBS for 0h 20m 0s at Room temperature.
--- Pause Point ---
After 1X PBS washes, gels can be stored in 1X PBS for up to one month at 4°C. Be sure to store gels in a sealed container to avoid evaporation. 4- or 6-well plates, or petri dishes are suggested for long term storage.
[OPTIONAL] Trimming and Cutting
Further Trimming and Cutting for Long-Term Storage
It can be convenient to trim and store the gels as smaller fragments that are immediately ready for library preparation. This can be done in an image guided way by using DAPI staining to identify and extract regions of interest.
If image guidance is not necessary, the gels can be trimmed as in Step 19.2.
DAPI Staining
Transfer gel to a suitable container for imaging, such as a 4-well or 6-well plate. The plate does not need to have a glass-bottom for this step.
Prepare ~3-5 mL of DAPI in 1X PBS. Recipe for 1 mL is below.
| A | B | C | D |
|---|---|---|---|
| Reagent | Stock Concentration | Volume | Final Concentration |
| PBS | 1X | 999 μL | 1X |
| DAPI | 1 mg/mL | 1 μL | 1 mg/L |
| Total | 1000 μL |
Stain gel with DAPI solution for 0h 15m 0s at Room temperature.
Wash gel with 1X PBS for 0h 10m 0s at Room temperature.
Imaging and Trimming/Cutting
Image gel on microscope using 4X objective in wide-field mode, using the imaging settings for DAPI.
Trim gel as appropriate using DAPI staining as a landmark. To cut the gel, use a thickness #2 (or #1.5) coverslip as a blade. After pushing the coverslip down (and holding in place), sweep a paintbrush alongside the coverslip to ensure the two sides are separated.
Additional Cutting and Storage
After trimming the gel, the interior can be cut into smaller sections. This can either be done on the microscope using DAPI as a landmark (as above), or without imaging at the bench.
Smaller gel sections can be transfered to individual PCR tubes in 1X PBS, and stored for up to one month at 4°C.

