Synthetic Procedure of Pinoresinol
Lisa.Stanley, Rui Katahira, Gregg T. Beckham
lignin model compounds
dimers
nuclear magnetic resonance
pinoresinol
lignin
synthesis
beta-beta linkage
Disclaimer
This work was authored by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. Funding provided by U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Bioenergy Technologies Office. The views expressed herein do not necessarily represent the views of the DOE or the U.S. Government.
Abstract
A direct understanding of the degradation reaction pathways of lignin polymers in biomass is difficult due to the complexity of lignin’s structure. To overcome the difficulty, simple lignin dimeric and trimeric model compounds which include typical lignin interunit linkages are useful to clarify reaction mechanisms. The following procedure describes the synthetic procedure of a β-β dimeric lignin model compound: pinoresinol. Lignin model compounds are useful for screening the effectiveness of catalysts and microoganisms. As well as determining the effect of a treatment on the lignin fraction, in particular the effect on the degree of depolymerization in the lignin polymer.
Before start
All glassware is dried in an oven set to 105ºC then cooled in a desiccator prior to use.
Steps
Synthetic Procedure
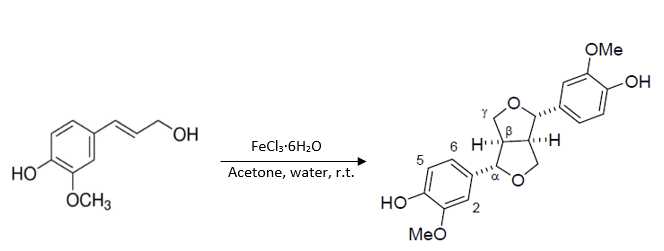
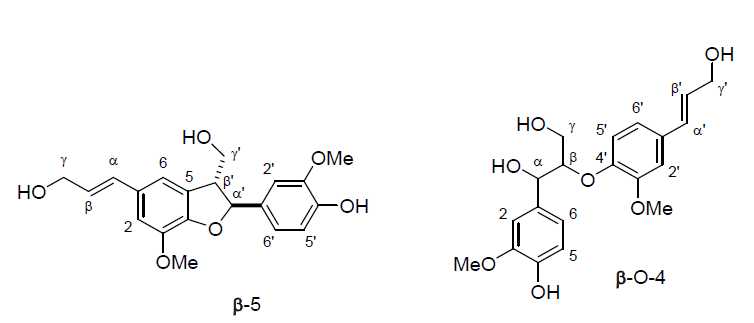
Coniferyl alcohol (2.0782g , 11.5 mmol) was dissolved in acetone (15mL ) then diluted with deionized (D.I.) water (70mL ). A solution of iron (III) chloride hexahydrate (3.4289g , 12.7 mmol, 1.1 eq) in D.I. water (30mL ) was then added to the reaction mixture. The mixture was stirred at room temperature for 1 hour. After an hour, the reaction was extracted five times with ethyl acetate (70mL x5). It was then washed with a saturated solution of brine (350mL ) [see Note 1], dried over sodium sulfate, and concentrated in vacuo .[1,2]The crude mixture was purified via flash chromatography to yield pinoresinol (0.4971 g, 24%) as well as β-O-4 (0.5507 g, 25%) and β-5 (0.261 g, 13%) dimer side products.
[1]
Purification
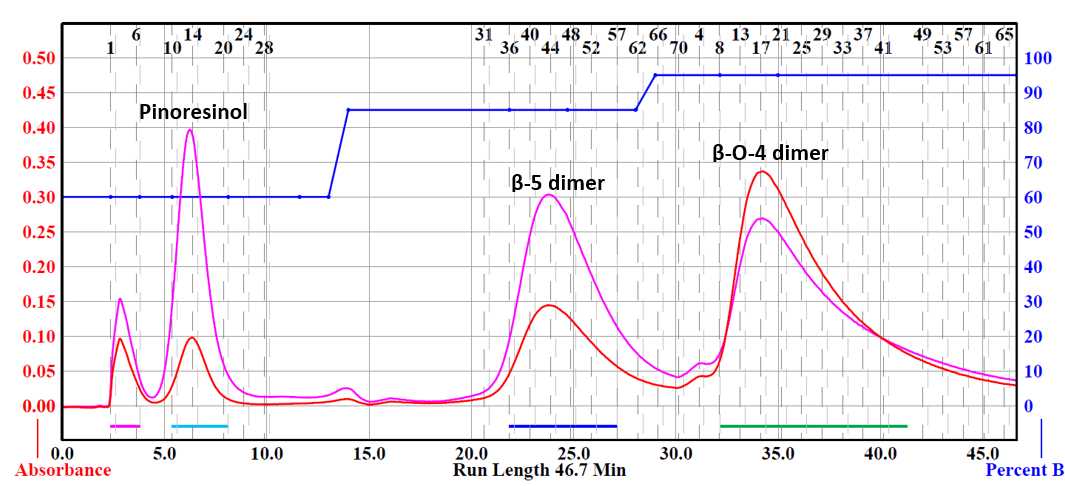
Flash chromatography was performed using a Teledyne Isco Combiflash® NextGen 300+. Collected fractions were determined using a UV detector with wavelengths set at 254 and 280 nm. Samples were prepared by dissolving the crude material in the smallest amount of compatible solvent. Silica gel (mesh size 70-230) was then added to adsorb the material. Excess solvent was vacuum evaporated and the sample was loaded into a RediSep® Rf 25 g sample cartridge (catalog # 69-3873-240).
Pinoresinol was purified via flash chromatography. Column used was a RediSep® Silver 80 g silica gel flash column (catalog # 69-2203-380). Solvent system was hexane (Solvent A) and ethyl acetate (Solvent B). Pinoresinol was collected starting five minutes into the run using a ratio of 60% ethyl acetate: 40% hexane. The β-5 dimer side product was collected starting twenty minutes into the run using a ratio of 85% ethyl acetate:15% hexane. The β-O-4 dimer side product was collected starting thirty-two minutes into the run using a ratio of 95% ethyl acetate:5% hexane.
NMR Spectroscopy
Nuclear magnetic resonance (NMR) spectra are acquired in suitable deuterated NMR solvent at 25°C on a Bruker AVANCE 400MHz spectrometer equipped with a 5 mm BBO probe. Chemical shifts (δ) are reported in ppm. 1H-NMR spectra are recorded with a relaxation delay of 1.0 s and an acquisition time of 4.09 s. The acquisition parameters for 13C-NMR include a 90˚ pulse width, a relaxation delay of 1.0 s, and an acquisition time of 1.36 s. Tetramethylsilane is used as a reference.
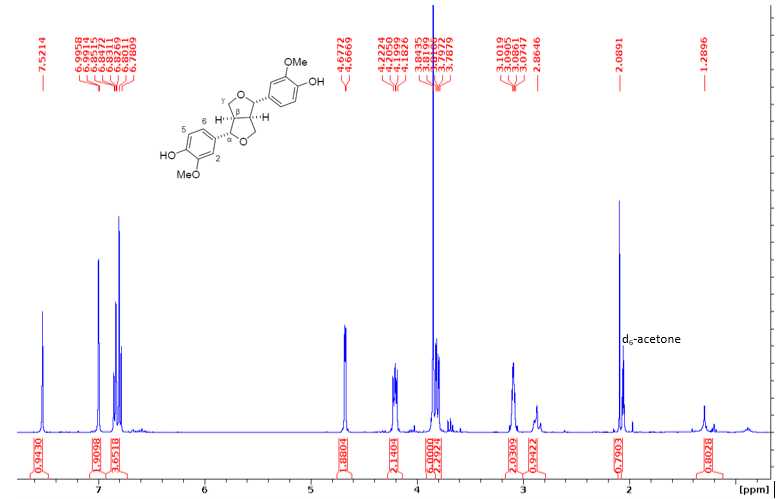
1H NMR (400 MHz, d6-acetone) δ 7.52 (s, 1H, ArOH), 6.99 (d, J=1.8 Hz, 2H, H2), 6.85-6.78 (m, J = 8.2, 1.7 Hz, 4H, H5/6), 4.67 (d, J = 4.1 Hz, 2H, H), 4.22 – 4.18 (m, J= 6.9, 2.0 Hz, 2H, H), 3.91 (s, 6H, OMe), 3.84 – 3.78 (m, 2H, H), 3.10 – 3.07 (m, J= 4.6, 1.8 Hz, 2H, H).
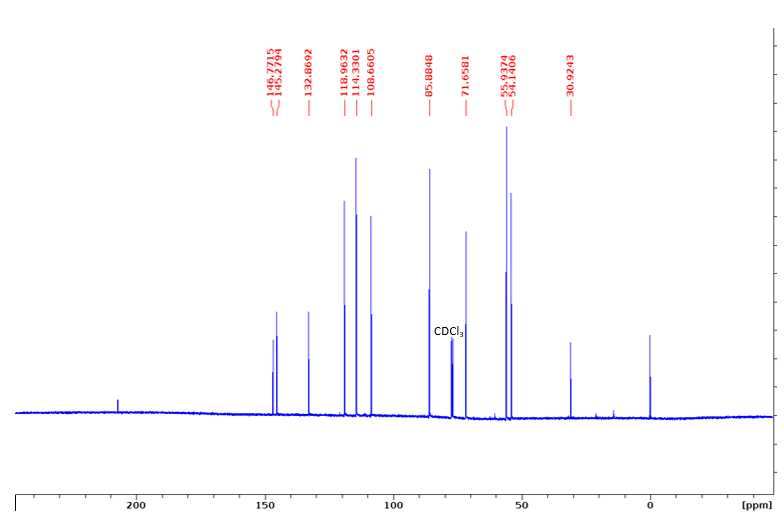
13C NMR (100 MHz, CDCl3): 146.77 (3), 145.28 (4), 132.87 (1), 118.96 (6), 114.33 (5), 108.66 (2), 85.88 (α), 71.66 (γ), 55.94 (OMe), 54.14 (β).
β-5 1H NMR (400 MHz, d6-acetone): δ 7.67 (s, 1H, ArOH), 6.94-6.85 (m, 5H, aromatic region), 6.40 (d, J=1.6 Hz, 1H, Hα), 6.18 (dt, J=9.2, 6.6 Hz, 1H, Hβ), 5.10 (d, J=9.3 Hz, 1H, Hα'), 4.31 (d, J=5.9 Hz, 2H, Hγ), 3.86 (s, 3H, OMe), 3.85 (s, 3H, OMe), 3.84 (m, 2H, Hγ'), 3.46 (m, 1H, Hβ').
β-O-4 1H NMR (400 MHz, d6-acetone): δ 7.42 (s, 1H, ArOH), 6.91-6.87 (m, 6H, aromatic region), 6.56-6.50 (m, 1H, Hα'), 6.34-6.31 (m, 1H, Hβ'), 4.91-4.87 (m, 1H, Hα), 4.53 (s, 2H, Hγ'), 4.22 (ddd, J=5.6, 3.6, 1.4 Hz, 0.5H, OHγ), 4.08 (dt, J=7.1 Hz, 0.5H, OHγ), 3.89-3.85 (m, 2H, Hγ), 3.82 (s, 3H OMe), 3.81 (s, 3H, OMe), 3.57-3.44 (m, 0.5H, OHα), 2.92 (dd, J=7.1, 5.6 Hz, 0.5H, OHγ'), 2.77 (dd, J=8.4, 5.6 Hz, 0.5H, OHγ').
[3]