Sonication of α-synuclein Fibrils for injection into the mouse brain
Vijay Singh, Marta Castellana-Cruz, Nunilo Cremades, Laura Volpicelli-Daley
Disclaimer
The protocols.io team notes that research involving animals and humans must be conducted according to internationally-accepted standards and should always have prior approval from an Institutional Ethics Committee or Board.
Abstract
Animal models that accurately recapitulate the accumulation of alpha-synuclein (α-syn) inclusions, progressive neurodegeneration of the nigrostriatal system and motor deficits can be useful tools for Parkinson's disease (PD) research. The preformed fibril (PFF) synucleinopathy model in rodents generally displays these PD-relevant features, however, the magnitude and predictability of these events is far from established. We therefore have optimized the sonication protocol of α-syn fibrils to ensure reliable, robust results. This protocol includes steps for sonication of PFFs on the day of injection into mice.
Before start
Steps
Sonicating α-synuclein Fibrils (PFFs)
Fill Qsonica Sonicator (Q700 Sonicator) water reservoir with about 900mL deionized water.
Turn on the heating system of the Q700 Sonicator and set the temperature at 10°C.
After the temperature inside Q700 Sonicator water reservoir is equilibrated to 10°C, thaw and pipette 25-22µL PFFs stored at -80°C and transfer to polystyrene sonication tube (Active Motif, Cat No. 53071) inside a biosafety cabinet. Screw the cap of the polystyrene sonication tube tightly after transferring, but avoid overtightening.
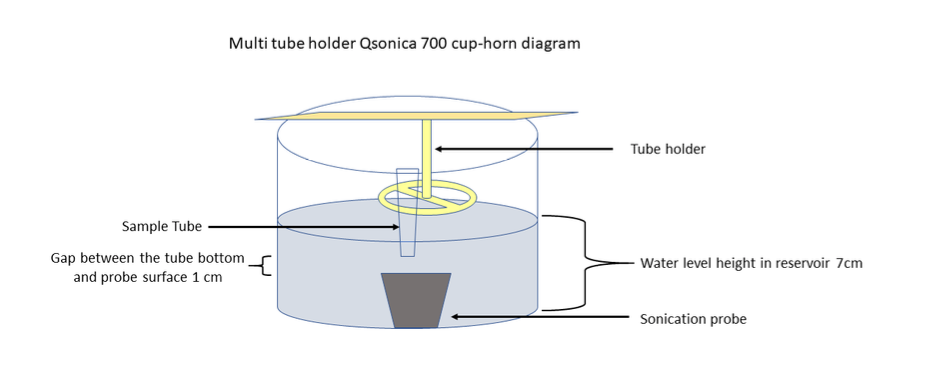
Fit the polystyrene sonication tube containing PFFs to the Q700 Sonicator tube holder and transfer it into the Q700 Sonicator water reservoir. Ensure the reservoir water level is maintained up to the level of the upper meniscus of the PFFs (PFFs: green in the figure below). Operate the sonication for 0h 15m 0s at an amplitude of 45%, with pulse on and off durations of 0h 0m 3s and 0h 0m 3s respectively, which should generate a power of around 110 watts for each pulse.
Take the sample tube after sonication and wipe it to remove any water from the reservoir. Spin the tube at 1000rpm,0h 0m 0s for a few seconds to settle PFF droplets inside. Inside the biosafety hood, remix the PFF sample by pipetting up and down 5 times, ensuring to avoid introducing bubbles. After remixing, re-sonicate the sample for 0h 7m 30s using the same sonication parameters as described above in step 4.
PFFs Injection
Inject the sonicated PFFs in the mouse brain as soon as possible within 8h 0m 0s . PFFs should be kept at room temperature until all mice are injected. The monomer, serving as a control, should be spun down at °C at 20000x g,0h 0m 0s for 0h 15m 0s to obtain supernatant for injection and then kept on ice until all mice are injected. Any leftover PFFs should be properly disposed of after SDS treatment as indicated earlier.

