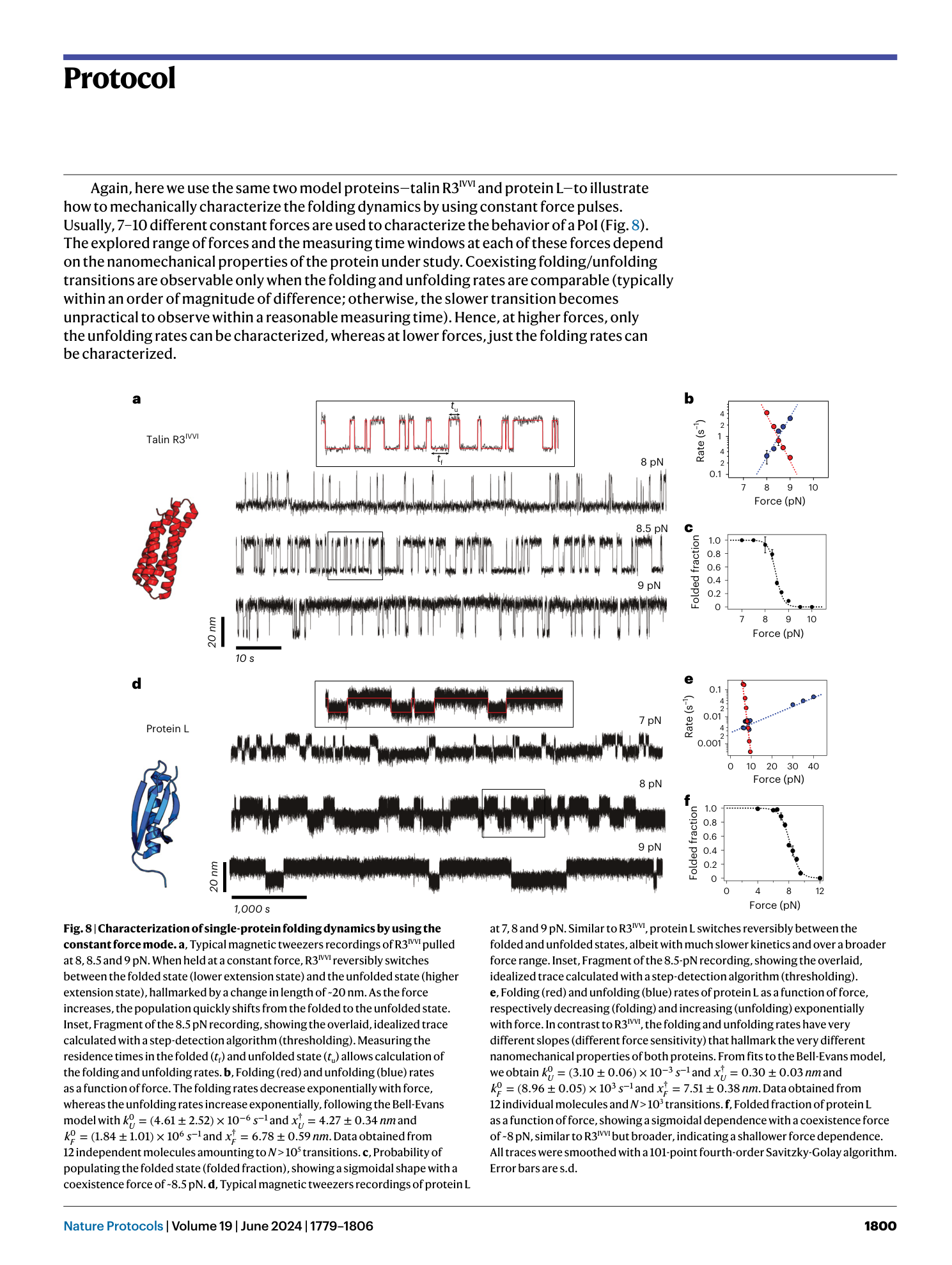
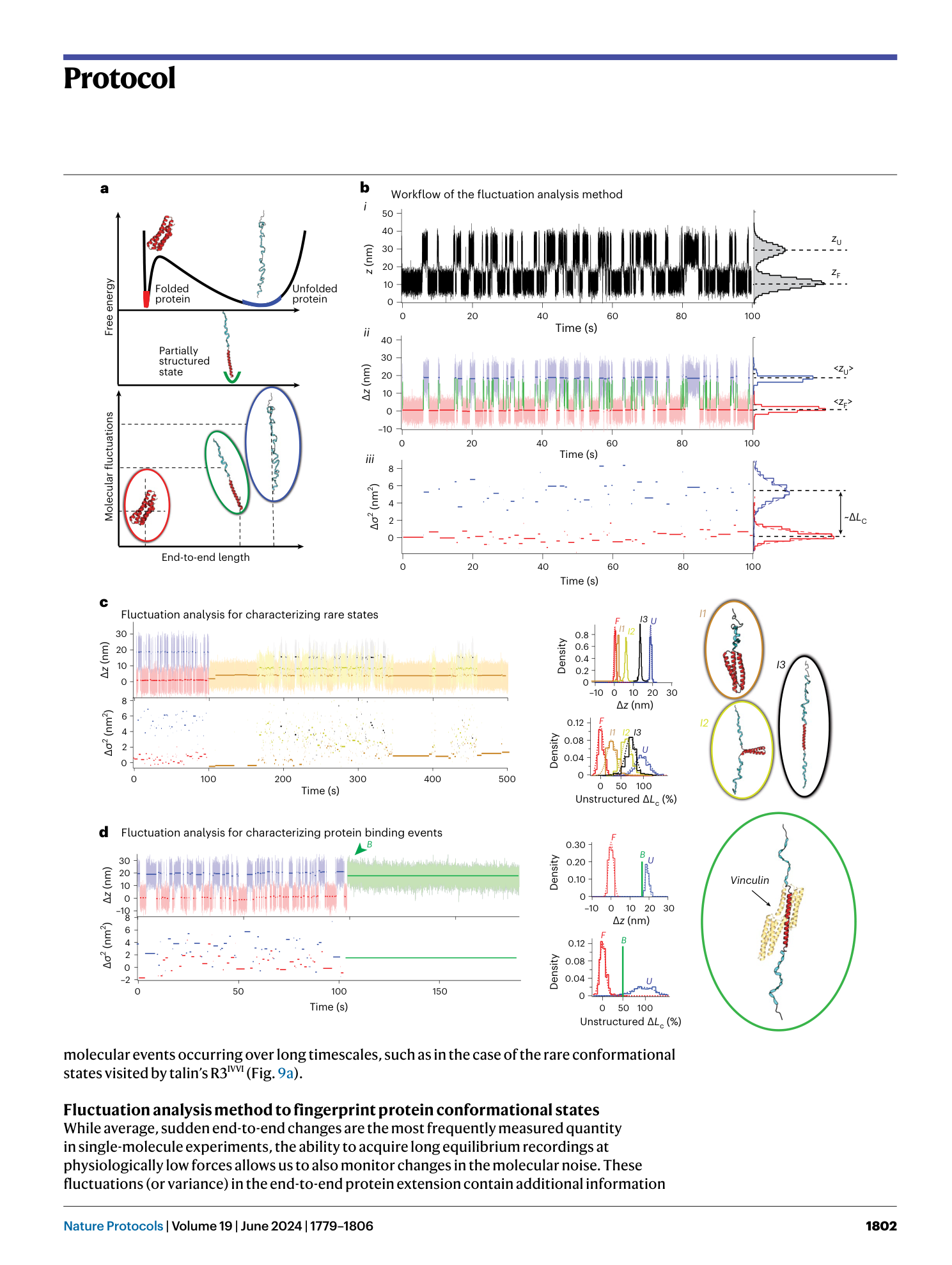
Single-molecule magnetic tweezers to probe the equilibrium dynamics of individual proteins at physiologically relevant forces and timescales
Rafael Tapia-Rojo, Marc Mora, Sergi Garcia-Manyes




















Extended
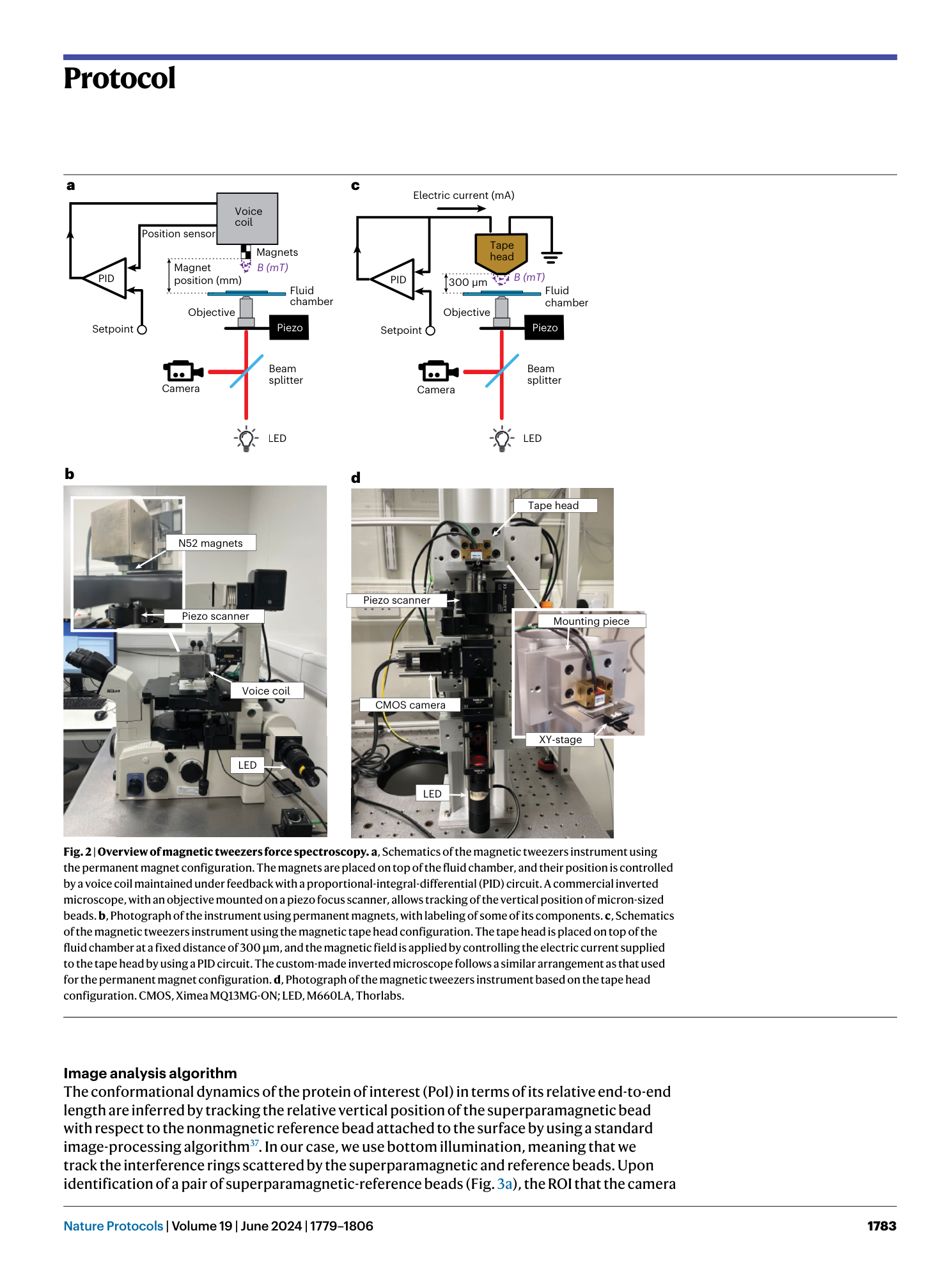
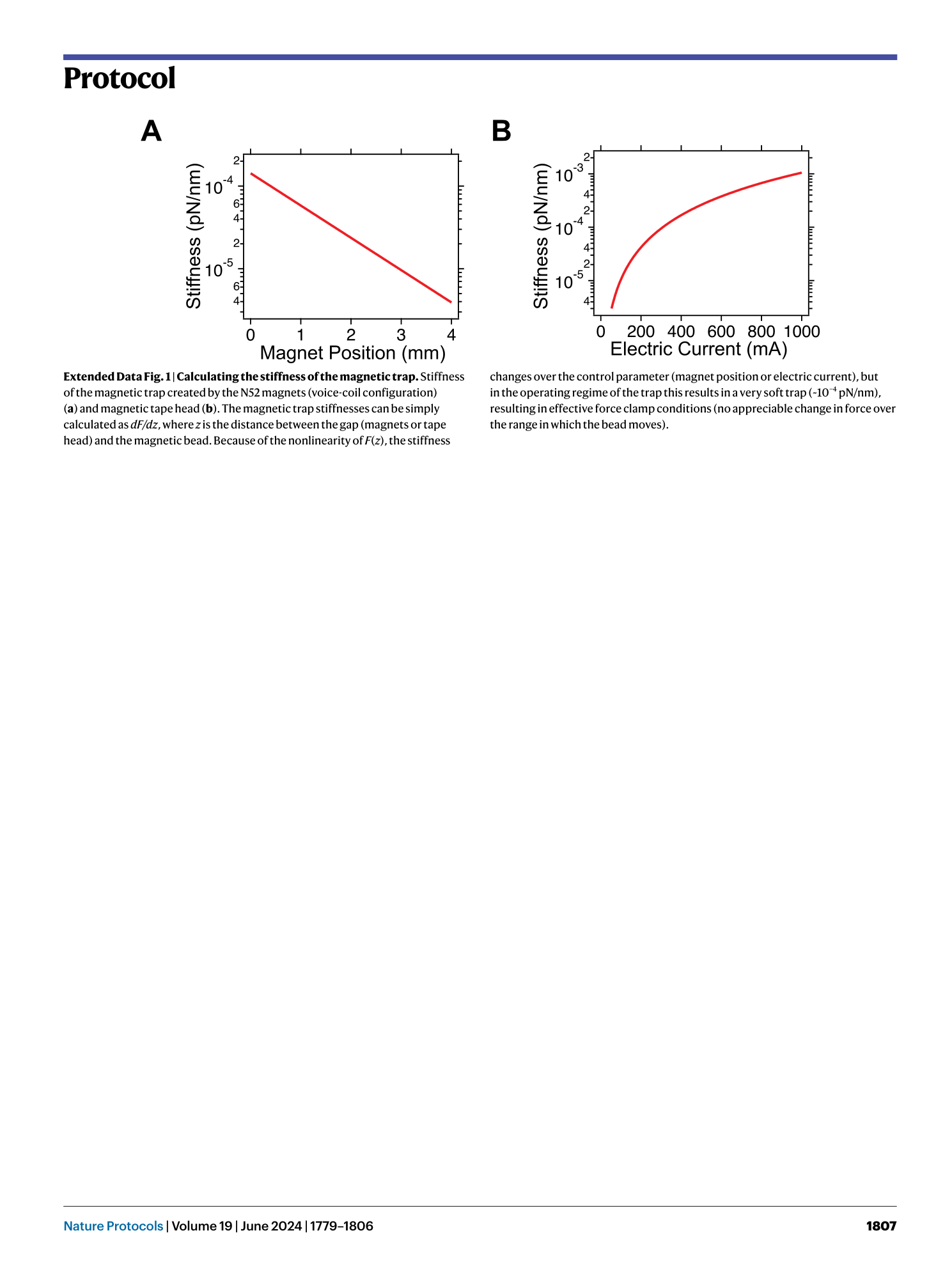
Extended Data Fig. 1 Calculating the stiffness of the magnetic trap.
Stiffness of the magnetic trap created by the N52 magnets (voice-coil configuration) ( a ) and magnetic tape head ( b ). The magnetic trap stiffnesses can be simply calculated as dF/dz , where z is the distance between the gap (magnets or tape head) and the magnetic bead. Because of the nonlinearity of F ( z ), the stiffness changes over the control parameter (magnet position or electric current), but in the operating regime of the trap this results in a very soft trap (~10 −4 pN/nm), resulting in effective force clamp conditions (no appreciable change in force over the range in which the bead moves).
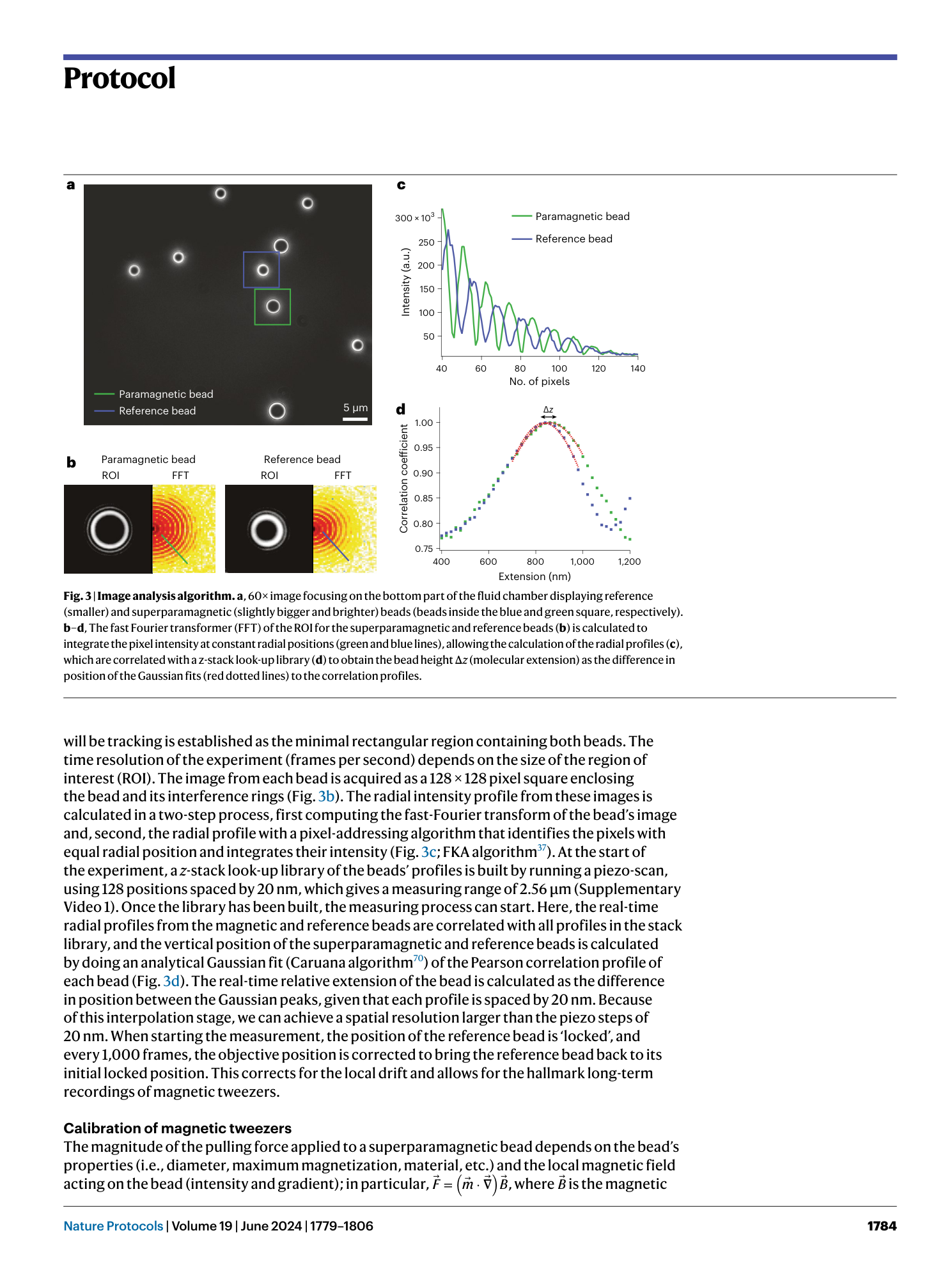
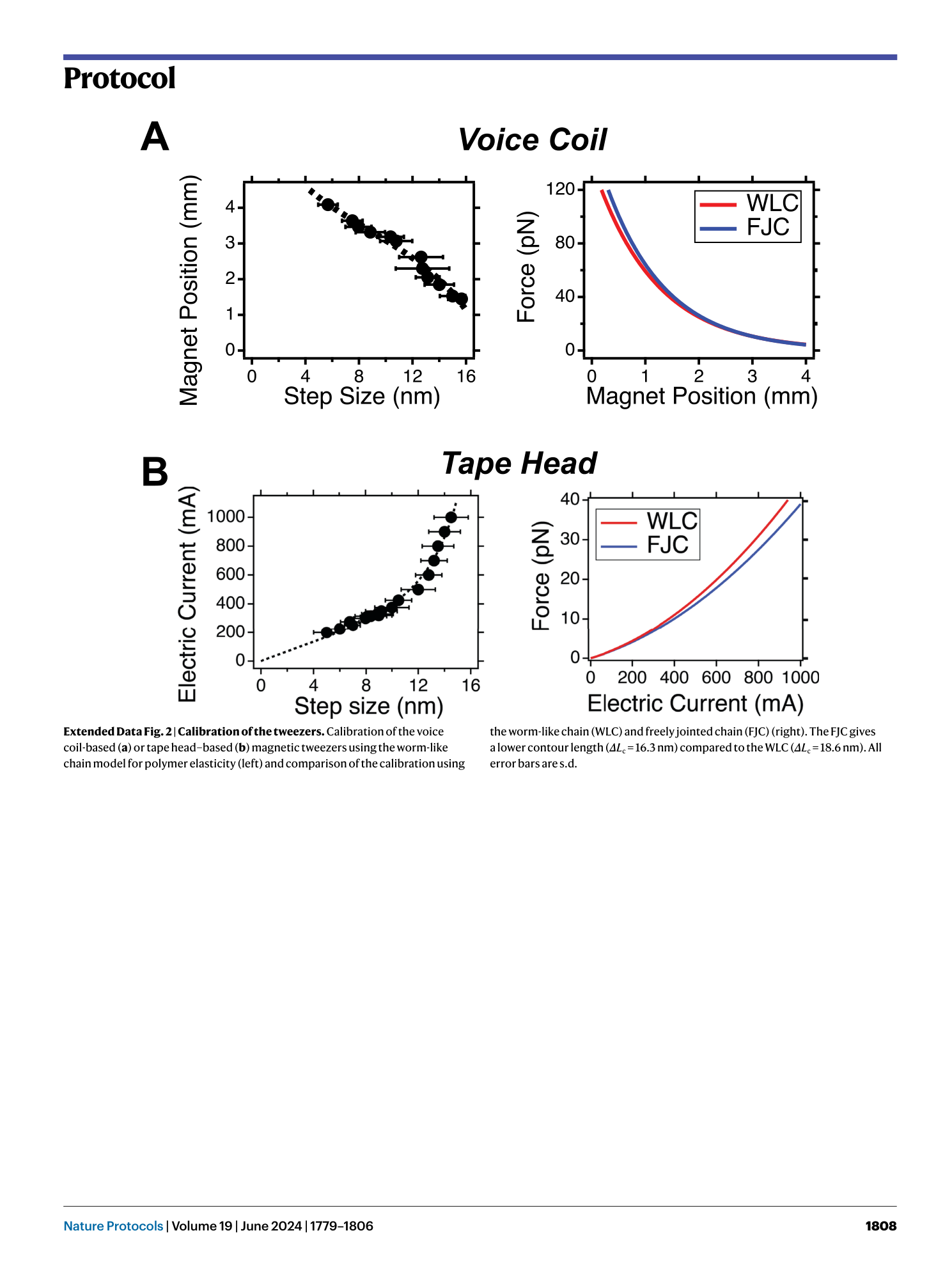
Extended Data Fig. 2 Calibration of the tweezers.
Calibration of the voice coil-based ( a ) or tape head–based ( b ) magnetic tweezers using the worm-like chain model for polymer elasticity (left) and comparison of the calibration using the worm-like chain (WLC) and freely jointed chain (FJC) (right). The FJC gives a lower contour length ( ΔL c = 16.3 nm) compared to the WLC ( ΔL c = 18.6 nm). All error bars are s.d.
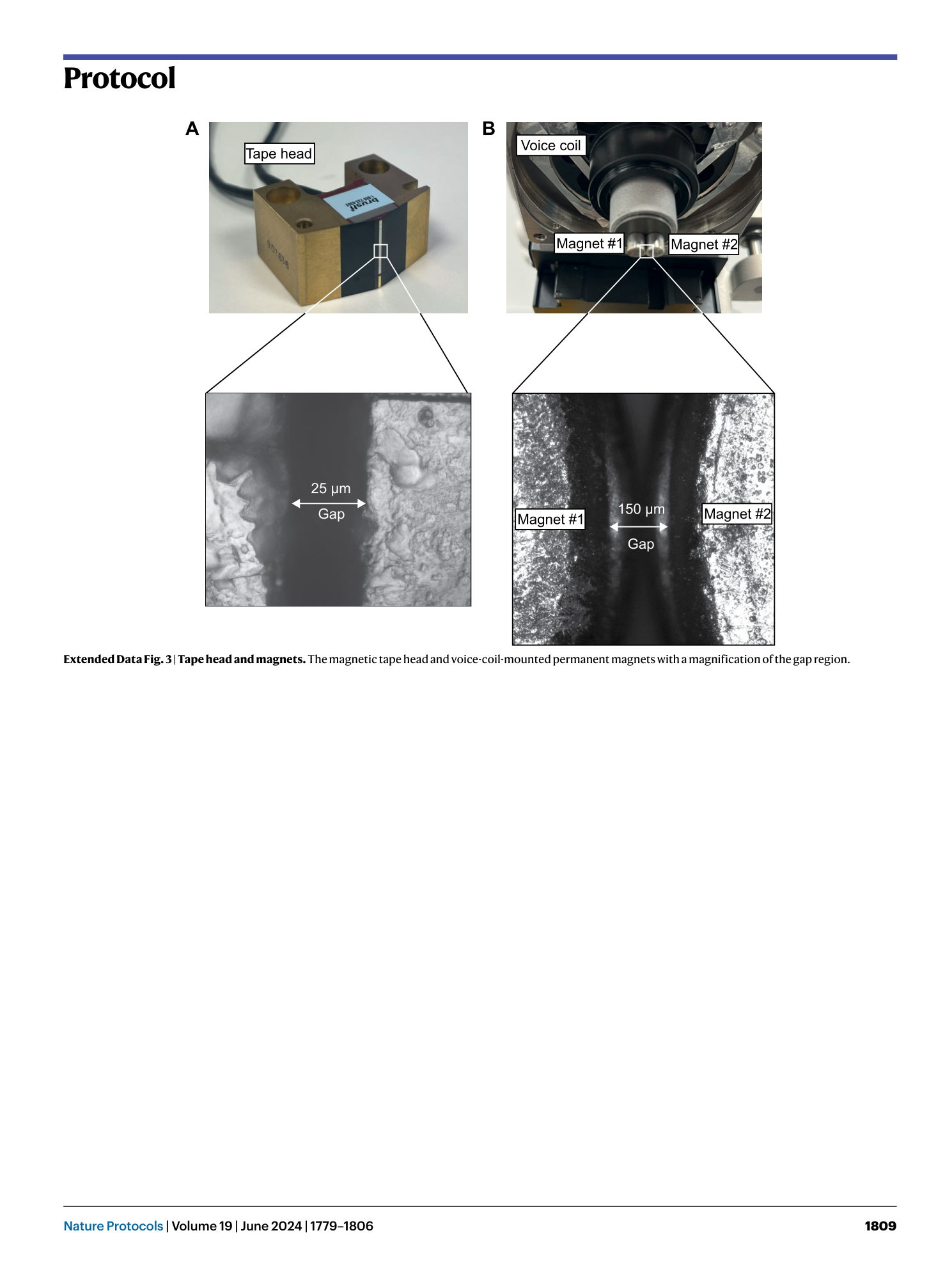
Extended Data Fig. 3 Tape head and magnets.
The magnetic tape head and voice-coil-mounted permanent magnets with a magnification of the gap region.
Supplementary information
Reporting Summary
Supplementary Data 1
Raw traces from talin R3 IVVI pulled at 1 pN/s and protein L pulled at 5 and 10 pN/s
Supplementary Data 2
Raw trace and fluctuation analysis of talin R3 IVVI pulled at 8.5 pN
Supplementary Videos 1–3
1, how to pull on a protein by using single-molecule magnetic tweezers; 2, how to assemble the fluid chambers; 3, how to calibrate the distance between the bottom glass cover slide and the magnets

