Seawater virome concentration with Vivaflow
Natascha Varona, Cynthia Silveira
Disclaimer
You can reuse the Vivaflow cassette multiple times for different samples by cleaning between samples. However, you should always check the filtrate for VLPs using fluorescence microscopy to ensure the cartridge is still intact (no viruses going through the filter membrane).
Abstract
Concentration of viral particles from seawater samples using a Vivaflow.
Steps
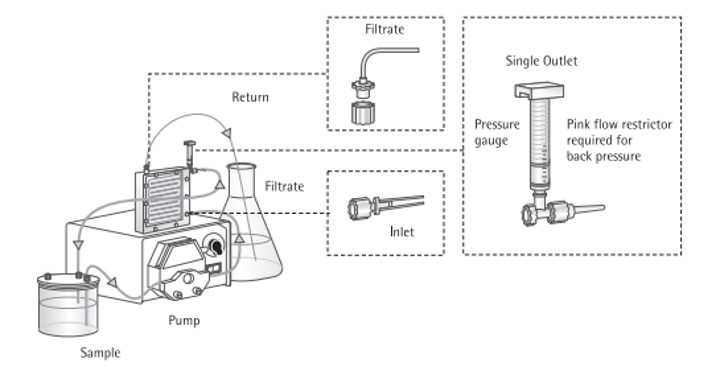
Virome collection
Collect 500mL - 2L of seawater (depending on microbial density) in a sterile container.
Wipe down outside of all masterflex tubing with 70% ethanol.
Insert one end of the tubing into the sample and feed it through the Masterflex pump.
Attach 0.22 µm (or 0.45 µm depending on the viral size fraction you want to concentrate) Sterivex to the other end of the tube using a luer lock adapter.
Turn the Masterflex on a low setting (between 2-3).
Keep the flowthrough for virome concentration.
Virome concentration
Before running the sample, rinse Vivaflow with 500mL of DI water by inserting a sample tube into a container with DI water and placing the filtrate and return tubes in a waste container.
Pump water at a rate of 200-400 mL/min.
After 400mL have passed into the waste, stop the pump, and check for leakage at connections.
Tighten if needed. Drain system. It is now ready for use.
Replace the DI water with the sample and collect the filtrate in a sterile container (this filtrate will be used for backflush later).
Move the return tubing to a waste container to rinse out residual DI-water before recirculating.
Pump liquid at a rate of 200-400 mL/min for a few seconds to ensure the DI water is removed and stop the pump.
Move the return tube to the sample container (note that the feed tube and return tube are now in the sample, this is necessary to recirculate and concentrate the sample).
Pump liquid at a rate of 200-400 mL/min. (If using a pressure indicator it should be approximately 2.5 bar).
When the sample container is nearly empty, remove the feed line, and collect the sample in a 50 mL conical tube. This usually contains about 30mL of the sample.
For a more complete recovery, preform a backflush.
Remove and screw off filtrate line.
Take about 20mL aliquot of the filtrate, and insert the feed tube into the filtrate.
Run the Vivaflow in reverse until the sample reaches about 50mL .
Add chloroform to your virome sample for a final concentration of 0.1% (v/v).
Example: 50 µL Chloroform for 50 mL of sample.
Samples can be stored at 4°C for later extraction.
Vivaflow clean up
Reattach the filtrate line and flush the system with 200mL of DI water with the filtrate going to the waste.
Place the feed, return and filtrate lines into a container with a cleaning solution of 250mL of 0.5millimolar (mM) NaOCl and 0.5Molarity (M) NaOH. (250mL DI-water + 133µL NaOCl + 5g NaOH)
Recirculate at 50-100 mL/min for 30 minutes.
Drain the system and recirculate 250mL of DI-water through the system for 5-10 minutes.
Drain and rinse system with a further 500mL of DI-water.
The system is now ready for the next sample, or for long term storage, fill the module with 10% ethanol and (in DI-water) store at 4°C .