Screening procedure to identify triazole-resistant Aspergillus spp. using agar plates
Juliê JA Alves
Abstract
The methodology of the screening procedure for detecting resistance to azoles in the genus Aspergillus spp. consists of an in house technique, with visual analysis of fungal growth on agar plates supplemented with azoles, Itraconazole at a concentration of 4 µg/ml, Voriconazole 2 µg/ml and Posaconazole 0.5 µg/ml. This protocol is based on the E.Def 10.1 from EUCAST
Before start
Separate all reagents and identify the plates
Steps
Preparation of antifungal solutions:
From the antifungal powder prepare stock solutions in DMSO, at concentrations at least 200 times higher than those tested on the agar plate
From the stock solution, prepare the working solutions at the following concentrations:
Itraconazole (ITZ) - 400 µg/ml,
Voriconazole (VRZ) - 200 µg/ml
Posaconazole (PSZ) - 50 µg/ml
Storage Temperature: -25 to -18º C (Freezer)
For calculations, use the formula:
Initial concentration x Initial volume = Final concentration x Final volume
| A | B | C | D | E | F |
|---|---|---|---|---|---|
| Stock Solution Concentration | Stock Solution Volume | Volume of DMSO | Working Solution Concentration | Agar Final Concentration | |
| µg/mL | µL | µL | µg/mL | µg/mL | |
| ITZ | 1600 | 2500 | 7500 | 400 | 4 |
| VRZ | 400 | 5000 | 5000 | 200 | 2 |
| PSZ | 400 | 1250 | 8750 | 50 | 0,5 |
Example: Preparation of 10 mL of working solution of each of the azoles from the stock solution
Preparation of the medium:
Weigh all reagents:
RPMI 1640 powder – 10.4g
D-Glucose 2% - 18g
Bacteriological agar – 20g
MOPS - 34.53g (0.165 M)
In a 1L becker, Add 900mL of distilled water and RPMI powder 10.4g, glucose 2% 18g, Bacto Agar 20g, MOPS 34.53g
Microwave 0h 5m 0s, little by little, stirring with a glass rod, until the solutes are completely dissolved
If necessary adjust with a solution of NaOH (10 M) to 7.0
Divide the medium into 4 Erlenmeyer flasks containing 250mL each
Autoclave the medium at 121º for 0h 15m 0s.
Preparation of the azole-containing plates:
Identify all the plates with the azole or "control"
Cool the agar down to 45°C
Add 2.5mL of each azole working solution (100% final concentration) to each of the vials and ensure proper mixing
The remaining antifungal free vial will be used for the growth control
Pour the agar from each vial into the respective plates
Preparation of the inoculum:
For inoculum suspensions use fresh and mature cultures incubated for at least 2–7 days
Make a diluent stock solution by adding 1 ml of Tween 20 to every 50 ml of distilled water
Working in the biological safety cabinet, add 3 ml of the water/Tween 20 solution to the 3-7 day slope. Tween 20 will help to put the Aspergillus conidia into suspension.
If necessary gently detach only the conidia from the culture with a handle until the solution becomes turbid
This conidial suspension can be used in case of retest
Use the residual solution from the tube from steps 17-20 to perform the next steps
Add 2mL of destilled water in a tube and transfer 1-2 drops of the residual solution with conidial
Or transfer 1-2 drops of the conidial suspension
In some cases you will need more drops, for Aspergillus flavus for example
Adjustment is made by adding more destilled water if the suspension is very turbid and more conidia if the turbidity is very low.
Inoculation and incubation of agar plates:
Dip a sterile cotton swab in the inoculum suspension and rub the entire surface of the control plate in a back-and-forth motion
Re-dip the cotton swab and rub the entire surface in the same way of each of the three remaining plates with the azoles
Incubate the plates at 35-37°C for 48h 0m 0s and observe growth.
Visual analysis score definition:
0: no visible growth
1: weak/minimal growth (such as >5 tiny colonies or confluent weak growth where isolate was inoculated (covering ≤ half of the plate)
2: clearly visible growth with hyphal extension, but not covering entire plate
3: prominent uninhibited growth covering most of the plate
Analyzing the results
The presence or absence of fungal growth on the surface of the plates will yield the following preliminary classification:
-
Azole susceptible isolate: growth on the azole-free plate and absence of growth on the others plates
-
Potentially an azole non-susceptible isolate: growth on the azole-free plate and on any of the other supplemented plates.
CitationThe control plate should always show growth (score definition = 3)The azole containing plates will show growth only if the isolate is potentially resistant to the azole. 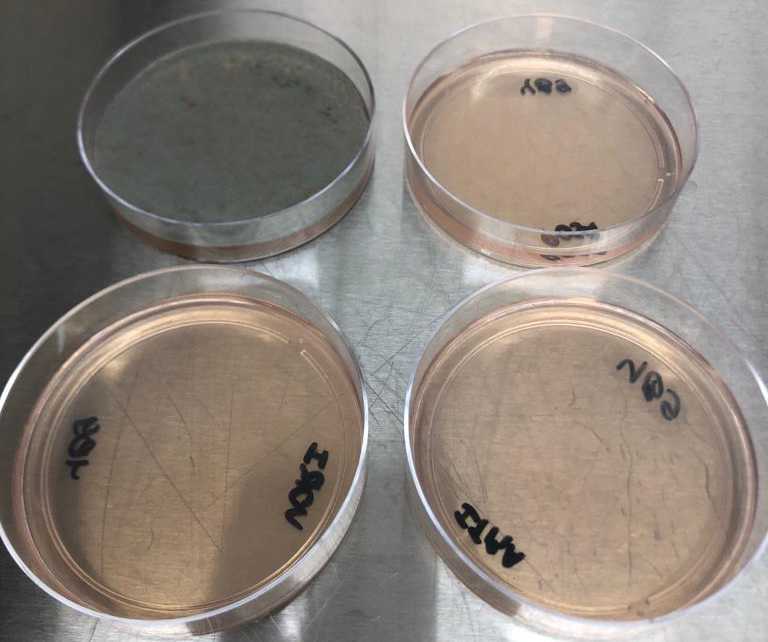
Azole susceptible isolate - Being the upper left control plate, without azole, upper right PSZ 0.5 µg/mL, lower left VRZ 2 µg/mL and lower right ITZ 4 µg/mL.