Quantitative detection of vitamin B12 in algae by bioassay and ICP-MS/MS
Sunnyjoy Dupuis, Stefan Schmollinger, Sabeeha S. Merchant
Disclaimer
E. coli strains were generously provided by Michi Taga.
Abstract
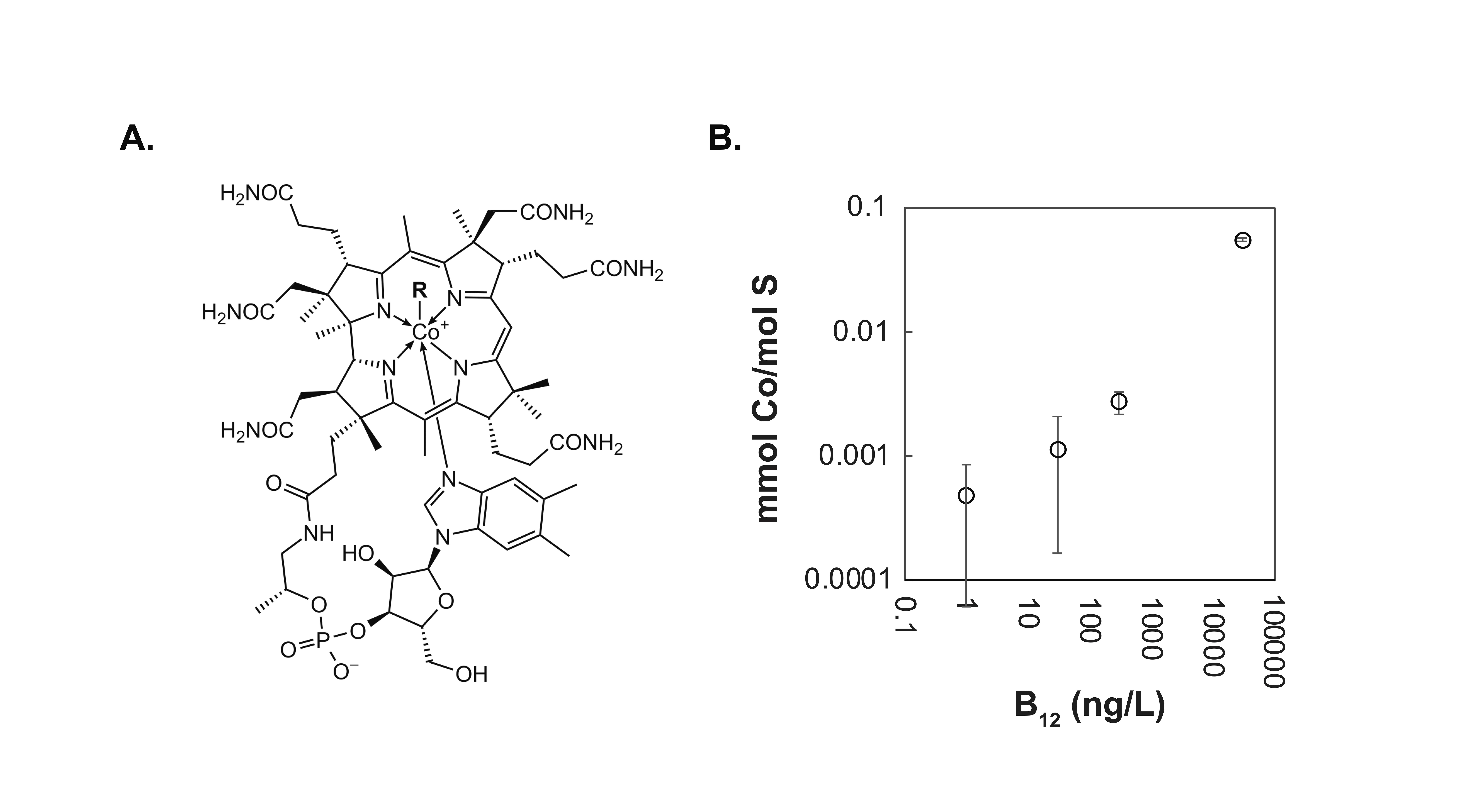
This protocol describes two methods for determining the amount of vitamin B12 present in the spent medium and cell lysate of algae cultures. The first method is a bioassay, adapted from Mok, Hallberg, & Taga (2022), which estimates the B12 concentration in solution from the growth of a B12-requiring Escherichia coli mutant. The second method uses the direct detection of cobalt via Inductively Coupled Plasma Mass Spectrometry (ICP-MS/MS) as a proxy for vitamin B12. We describe the preparation of spent medium and cell extract fractions from the chlorophyte alga Chlamydomonas reinhardtii for each method, preparation of standard cyanocobalamin solutions, and the correlation between cobalt and cyanocobalamin in algal cells.
We thank Michi Taga and Alison Smith for their guidance in optimizing the bioassay for C. reinhardtii .
Steps
E. coli B12 Bioassay
Prepare cell culture fractions:
Collect 2mL culture into a 2 ml screw-cap tube.
Centrifuge at >8000rcf. Quickly transfer 950µL of the spent medium supernatant to each of two 1.5 ml screw-cap tubes without disturbing the pellet.
To wash the pellet, resuspend in 1mL 0.85% NaCl, then centrifuge again at >8000rcf and discard the supernatant.
Resuspend the pellet in 1.9mL 0.85% NaCl.
Boil both cell suspension and spent medium fractions at 100°C for 0h 10m 0s to extract B12 from the cells.
Centrifuge all samples at >8000rcf,4°C.
Transfer the supernatant into convenient portions (2 or more) for downstream testing.
Flash freeze all samples in liquid nitrogen to store at -80°C.
Prepare cyanocobalamin (CNCbl) and methionine standards:
Prepare a series of CNCbl standards up to 10X the target concentration in purified water (Millipore) by serial dilution. Aliquot at least 250µL of each standard into screw-cap tubes.
Prepare a series of 1mL methionine standards to 10X the target concentration in milliQ H2O.
Boil standards at 100°C for 0h 10m 0s.

Centrifuge the standards at >8000rcf,4°C.
Flash freeze the standards in liquid nitrogen to store at -80°C.
Conduct the bioassay:
Inoculate starter cultures from single colonies of Δ metE and Δ metE Δ metH mutant strains from LB agar plates into2mL of M9 + 0.2% glucose minimal medium with 1 mg/mL methionine. Grow at 37°C 250rpm for 24h 0m 0s to saturation.
Generate pre-cultures by transferring 1% (vol/vol) of the saturated starter cultures into fresh 2mL M9 + 0.2% glucose minimal medium with 1 mg/mL methionine and grow at 37°C 250rpm for 24h 0m 0s to saturation.
On the day of the assay, prepare bioassay cultures: add 1mL of 2X M9 glucose, the desired amount of your sample or standard, and sterile Millipore water up to 2mL final volume.
The following control and test cultures should be included:
- Sample to be inoculated with Δ metE
- Sample to be inoculated with Δ metE Δ metH
- M9 medium alone without B12 or methionine supplementation for each E. coli strain (important to test efficacy of E. coli innocula washing)
- B12 and methionine standard series for each E. coli strain (important to include with every bioassay you perform, as maximum OD600 of strains can vary slightly from assay to assay)
- Controls for the highest concentrations of B12 and methionine in the standard series, and for one unknown sample, that will not be inoculated with E. coli (important to test for contamination or failure to kill study species during sample preparation)
Note
Complete this step prior to harvesting E. coli pre-cultures.
Next, harvest 2 mL of each E. coli pre-culture in snap-cap tubes and pellet by centrifuging at 10000rcf. Discard the supernatant.
Resuspend in 1mL sterile 0.85% NaCl, and repeat this wash twice more.
Resuspend washed pellet in 1mL 0.85% NaCl then measure OD600.
Use washed cells to inoculate the bioassay tubes at a starting OD600 of 0.01.
Incubate cells at 37°C 250rpm for 24h 0m 0s, then read final OD600.
Establish a standard curve that correlates the provided B12 and methionine concentrations in the standards to the OD600 of each E. coli strain E. coli strain. Determine the amount of B12 and methionine in your unknown samples by comparing the OD600 reached by each strain to the standard curves.
Sample preparation for ICP-MS Detection of B12-Derived Cobalt
Collect and wash samples:
Collect 1x108 cells in a 50ml falcon tube.
Centrifuge samples 3500rpm. Quickly transfer the supernatant to a clean tube for spent medium analysis.
Resuspend the pellet in 5-10mLof 1mM EDTA pH 8.0 (Washing Buffer 1), then fill up to 50mL.
Repeat wash step: centrifuge samples 3500rpm. Quickly discard the supernatant, then resuspend pellet in 5-10mLof Washing Buffer 1, and fill up to 50mL.
Wash cells again: centrifuge samples 3500rpm. Quickly discard the supernatant, then resuspend pellet in 1mLof Washing Buffer 1, then transfer to a 15 ml Falcon tube. To ensure all cells have been collected, add 5mL more Washing Buffer 1 to the 50 ml Falcon tube and transfer to the same 15 ml Falcon tube.
Centrifuge samples 3500rpm. Quickly discard the supernatant, then resuspend the pellet in 2-5mL of Millipore water, then fill up to 10mL of Millipore water.
Centrifuge samples 3500rpm. Quickly discard the supernatant. Then centrifuge samples 3500rpm, and remove the remaining supernatant completely using a filtered pipette tip.
Store the dry pellet and the spent medium at -20°C until further processing.
Digest cell pellet samples:
Thaw pellet at 65Room temperature, then centrifuge again 3500rpm to compact the pellet.
Carefully overlay the pellet with 286µL trace metal grade nitric acid (we use Fischer Chemical Trace Metal Grade Nitric Acid, Catalogue Number A509-P212).
Digest samples for 24h 0m 0s at 65Room temperature, then incubate for 2h 0m 0s at 65°C.
Add 9.5mL Millipore water for 2 % HNO3 final concentration. Vortex the sample thoroughly.
Samples are now ready for ICP-MS.
Prepare spent media samples: transfer 2mL spent medium to a 15ml Falcon tube, and add 5mLof 2.8% HNO3 for 2% final concentration. Spent media samples are now ready for ICP-MS.
Prepare


