Quantifying % Cover with ImageJ: An Analysis Tool for Image-based Assessments in Grazing Experiment Studies
Ramiro Logares, Clara Maria Arboleda-Baena, Claudia Pareja, Rodrigo De La Iglesia, Sergio navarrate, Javiera Poblete, Eric Berlow, Hugo Sarmento
Disclaimer
The authors declare no conflict of interest.
Abstract
Protocol used in the article “Unifying microorganisms and macrograzers in rocky shore ecological networks: trophic and non-trophic effects on microbial community”.
Steps
PROTOCOL
We analyzed 10 rocks for each treatment, examining the effects of epilithic biofilm grazing by five different species: the chiton Chiton granosus (Frembly 1828), the littorinid Echinolittorina peruviana (Lamarck 1822), the keyhole limpet Fissurella crassa (Lamarck 1822), the scurrinid limpet Scurria araucana (d'Orbigny 1839), and the pulmonate limpet Siphonaria lessonii (Blainville 1827). The experiment lasted 24 hours when animals were carefully removed. Within 24 hours, all 10 replicates rocks for each treatment were photographed and the biofilm cover was analyzed using the software Image J with the following protocol.
We captured three images of each rock: 1) before biofilm colonization ( R : rock), 2) after epilithic biofilm colonization ( B : biofilm), and 3) after the grazing experiment conducted by the five species ( G : grazing) ( Figure 1 ). In total, we analyzed 50 rocks, resulting in a collection of 150 pictures. To provide scale and reference, all the pictures were taken with a ruler positioned alongside the rocks.
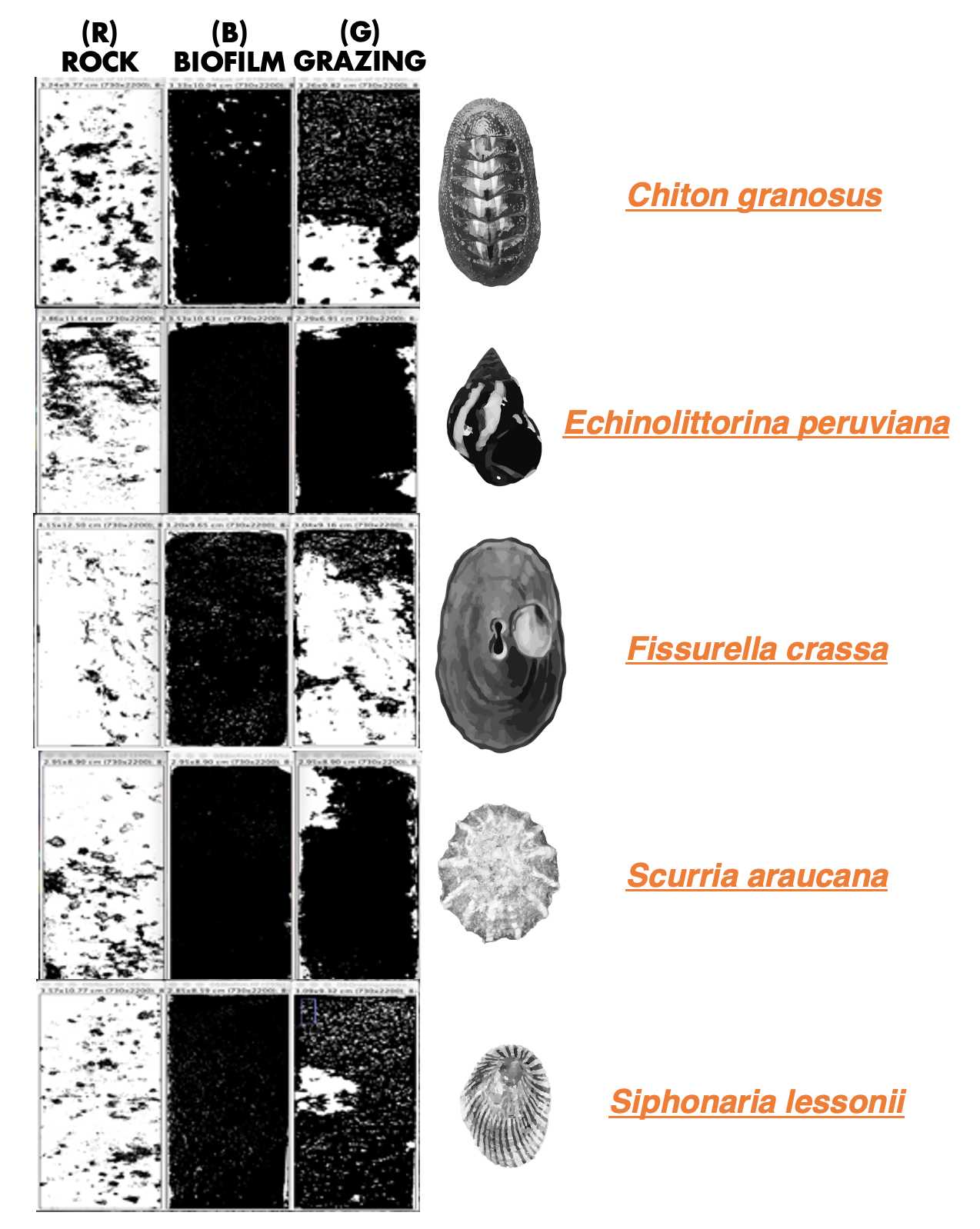
We developed the following ImageJ protocol for each picture (R, B, and G):
A. Select the "Straight" tool and mark 1 cm on the ruler in the photograph.
B. Go to "Analyze > Set Scale" and adjust the measurements using "known distance: 1" and "Unit of length: cm". Press the "Ok" button.
C. Crop the image to remove the background, ensuring only the rock is visible. Locate the "Rectangle" tool in the software's toolbar and select it. Use the "Rectangle" tool to draw a rectangle around the perimeter of the rock, ensuring that only the rock is enclosed within the shape. Then select "Image > Crop". The rock should be perfectly vertical and aligned in the same direction as the three pictures of each treatment (R, B, and G).
D. Adjust the image size by selecting "Image > Adjust > Size" and entering the values: 730 width / 2200 Height (pixels). Disable the option "Constrain aspect ratio".
E. Save the photograph as a ".tif" file using the code "Species Treatment + replicate number" + picture specification (R, B, or G) .
To analyze a single rock, follow these steps for each of the three pictures (R, B, and G):
F. Select the three pictures of one rock (R, B, and G) saved as .tif.
G. Convert each picture to 8-bit format by selecting "Image > Type > 8-bit".
H. Choose "Image > Adjust > Threshold". Select the "Red" channel and choose the "Default" option. Use the color red to define the biofilm or the darkest point on the rock. Make sure to disable the option "Dark background" to accurately measure the desired area.
*Recommendation: Keep the three pictures close by to assist in identifying the biofilm and distinguishing it from the dark minerals on the rock.
I. Proceed to "Analyze > Analyze Particles". Set the following parameters: Size (cm^2): 0 - infinity; Circularity: 0.00 - 1.00; Show: Nothing; Select: "Display results" and "Summarize".
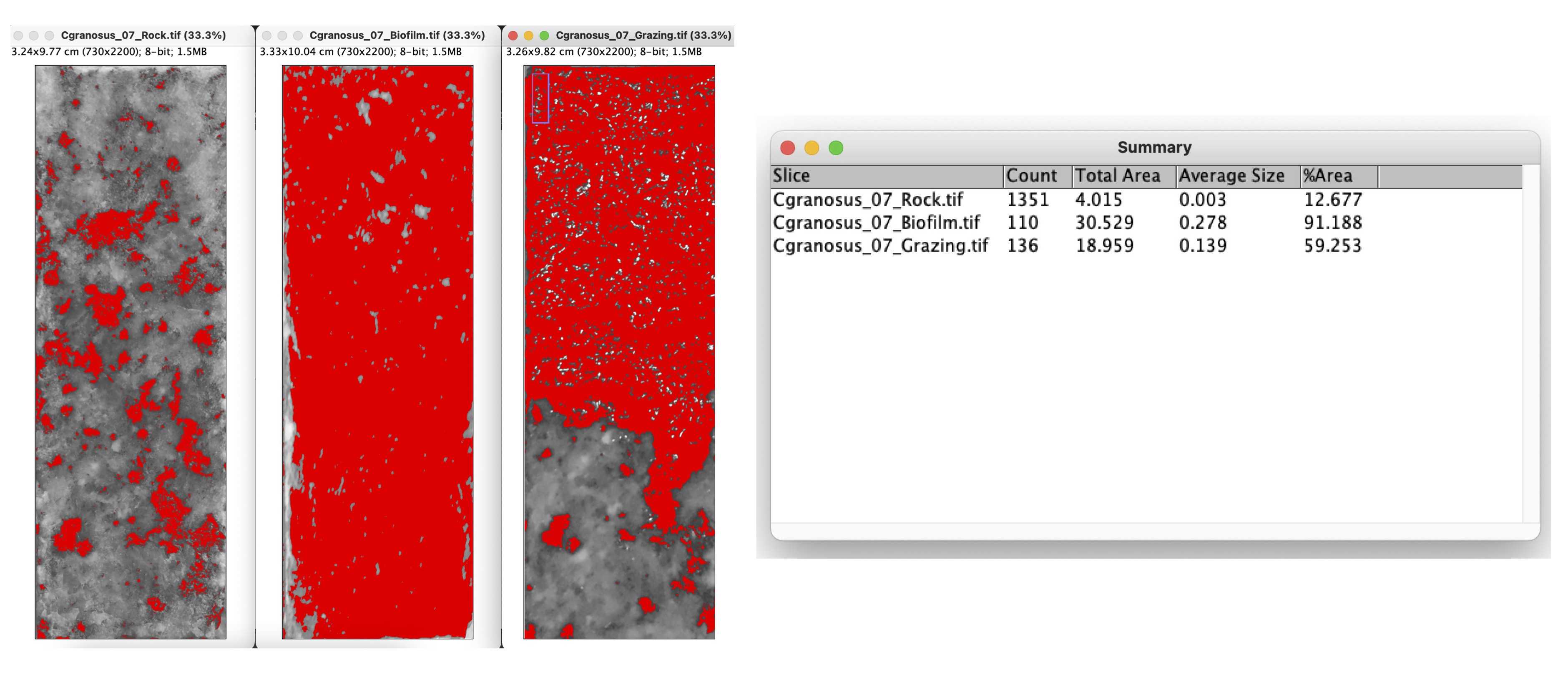
J. Copy the results into a table with columns for R , B , and G .
Please note that these steps should be repeated for each individual rock, considering the corresponding set of R , B , and G pictures.
The grazing percentage was determined by subtracting the value obtained from the biofilm picture ( B ) from the result of the grazing picture ( G ), and then further subtracting the background value obtained from the rock picture ( R ).
To assess the reproducibility of the method, we involved two different analysts. We calculated the average of the data generated by each analyst and performed a Welch's t-test (unequal variances t-test) to compare the results. The obtained p-value > 0.05, indicates that there were no significant differences in the analysis between the two analysts. Thus, the method demonstrates reproducibility.

