Preparing Combined Indexed Primer Plates (IDT Standard) for the PacBio Sequel2 - Sequel Dual Indices
André M Comeau, Gina V Filloramo
Abstract
The preparation of diluted combined (F+R) IDT working primer stocks of PacBio Dual Index primers for use in IMR PCR preps.
Steps
Order Primers
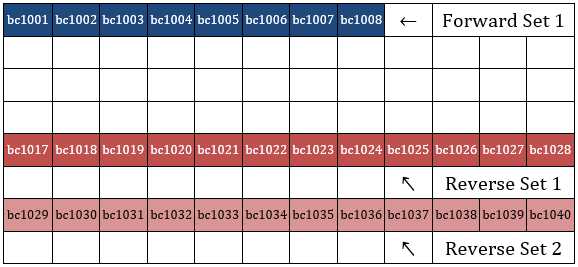
Use our Excel template (PacBio-CDI-16bp-customfusionprimers-template.xlsx) to copy existing full-length 16S/18S/ITS primers or to design your own custom gene primers with the proper PacBio indices. No special synthesis type (unlike longer Illumina fusion primers) is needed for these primers as they are close to the length of standard PCR primers. Order the indexed primers at 25nanomolar (nM) scale in deep-well plates (DWP); one set per 96-well plate, arranged as follows, leaving blank rows in between sets:
Prepare Archival Stocks
Once arrived, do a short spin of the plate in case lyophilized material was dislodged, then add 500µL of PCR-grade water to each well containing the primers in order to reconstitute them at a concentration of 50micromolar (µM) (1/2th the typical 100 µM working stock concentration for primers). Mix well by pipetting up and down at least 3 times and seal the plate with Bio-Rad film. Alternatively, the plate is sealed with Bio-Rad film and mixed well by vortexing it on a benchtop vortex for 0h 0m 30s and then doing a short spin at approx. 500rpm. We have found that these primers usually need a significant incubation time for the lyophilized pellets to re-suspend well – we typically leave them overnight at 4°C before continuing.
Prepare Intermediate Dilution Plate
Prepare a 10micromolar (µM) Intermediate Dilution plate of the Archival Stock plate above by pipetting 352µL of PCR-grade water into each corresponding well of a 96-well DWP from a sterile reservoir. Working by row and changing tips each time, transfer88µL of reconstituted primer from above into each well of each corresponding row, mixing well by pipetting (final volume of 440 µL which will be enough use the F1 primer 3 times to make working plates below [R primers will last longer]). The idea is to "stamp/copy" the exact layout of the above Archival Stock plate here into the Intermediate Dilution - this would normally mean, then, only Rows 1 (A1-A8 = F1 primers), 5 (E1-E12 = R1 primers) and 7 (G1-G12 = R2 primers) would be present in this new diluted plate. Seal the Archival Stock plate with PCR film and store at -20°C.
Prepare Combined Working Stocks
Prepare the combined 1micromolar (µM) working stock F1R1 Primer Plate by pipetting 216µL of PCR-grade water into each well of an empty 96-well DWP from a sterile reservoir. Rotate the above Intermediate Dilution primer plate 90° clockwise and align it so that the 8 occupied wells (= 8 different F1 indices) of Row 1 line up with the 8 rows of the new working stock plate. Working by column and keeping the same set of tips, transfer 12µL of reconstituted primer into each well of each column, mixing well by pipetting.
Now align the deep-well primer plate horizontally (normal orientation) so that the 12 occupied wells (= 12 different R1 indices) of Row 5 line up with the 12 columns of the working stock plate. Working by row and changing tips each time, transfer 12µL of reconstituted primer into each well of each row, mixing well by pipetting. Once complete, the resulting plate will have enough primer for 30 PCR plates (8 µL combined F+R per rxn × 30 = 240 µL). Seal the plate with PCR film and store at -20°C.
Prepare the combined 1micromolar (µM) working stock F1R2 Primer Plate by repeating Step 4, but using Row 1 (=F1) and Row 7 (=R2) instead.
Once all aliquoting is complete, seal the DWPs with PCR film and archive at -20°C until new aliquots are required (minimized freeze-thaw cycles).

