Preparation of acute midbrain slices for patch-clamp recordings
Oriol Pavon Arocas, Tiago Branco
Mouse
Slice electrophysiology
Patch-Clamp
Periaqueductal Gray
Neuroscience
Acute Brain Slices
Midbrain
Disclaimer
This work was funded by a Wellcome Senior Research Fellowship (214352/Z/18/Z) and a Sainsbury Wellcome Centre Core Grant from the Gatsby Charitable Foundation and Wellcome (090843/F/09/Z) (T.B.), and by a UCL Wellcome 4-year PhD Programme in Neuroscience Fellowship (203729/Z/16/Z) (O.P.A.).
We would like to thank Nicolas Wanaverbecq, Annalisa Scimemi, Vanessa Stempel, Yaara Lefler, Yu Lin Tan, Simon Weiler, Sara Mederos, and Hedi Young for their help and advice prior to the development of this protocol. We would also like to thank Robb Barrett from the Fabrications Lab at SWC for designing the 3D printed piece for the recovery chamber.
Abstract
This protocol describes two different approaches to prepare acute midbrain slices from young adult mice for slice electrophysiology experiments. It also provides general advice for troubleshooting common issues leading to suboptimal slice quality, as well as some suggestions to ensure good maintenance of a patch-clamp rig.
Before start
Ensure any animal work is carried out following the legislation and protocols in place at your home institution.
Steps
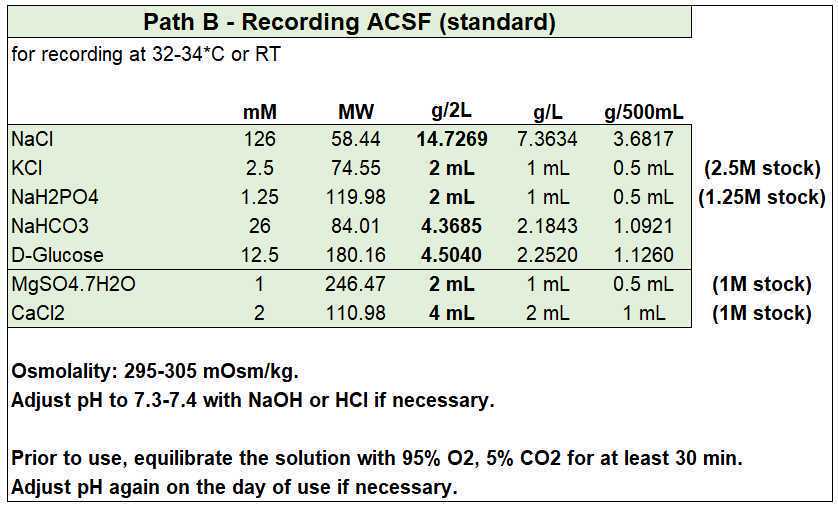
Preparation of stock solutions
[PATH A - Decapitation under terminal isoflurane anaesthesia]
This version of the protocol follows the first of two alternative slicing methods, which briefly consists on decapitation under terminal isoflurane anaesthesia, brain extraction and slicing in ice-cold slicing ACSF, incubation in slicing ACSF for 30 min at 35°C, followed by incubation in recording ACSF for 30 min at RT, and finally transferral to the recording chamber perfused with recording ACSF at 32-34°C or RT.
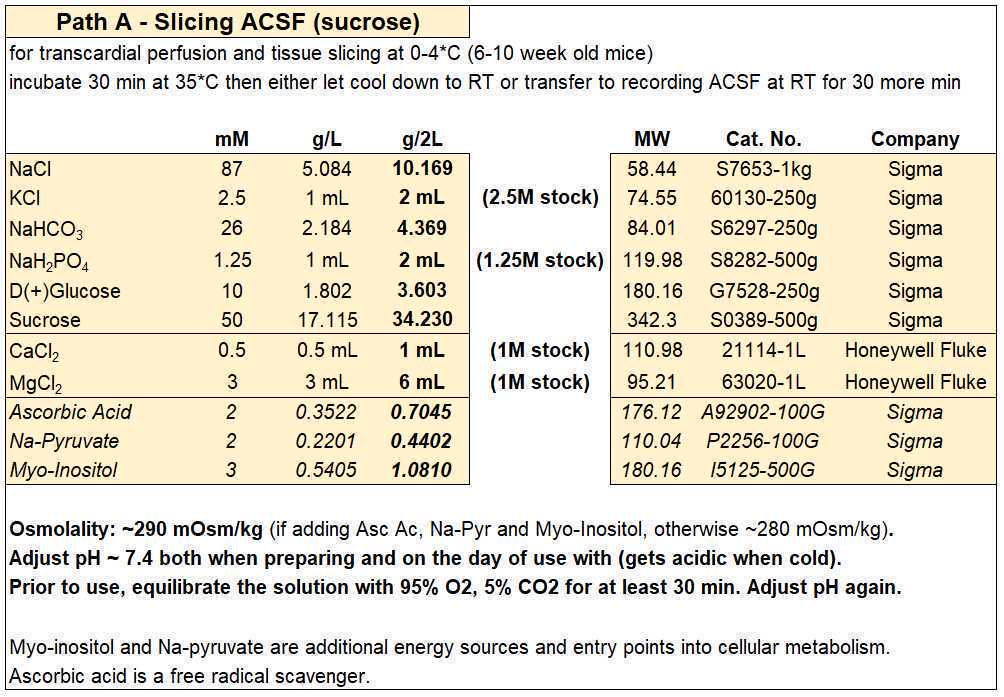
Slicing ACSF (sucrose)
- For each experiment you will need ~500 mL (~150 mL for the first recovery chamber and ~350 mL to make the slush to be used for slicing steps), so by making 2 L on Monday you should have enough solution for four days of experiments.
- Slicing ACSF (in mM): 87 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 10 D-glucose, 50 sucrose, 0.5 CaCl2, 3 MgCl2 (equilibrated with 95% O2 and 5% CO2), with an osmolality of ~280 mOsm/kg.
- Optionally, you can add (in mM) 2 Ascorbic Acid, 2 Na-Pyruvate, and 3 Myo-Inositol. Ascorbic acid is a free radical scavenger, while Na-Pyruvate and Myo-Inositol are additional energy sources and entry points into cellular metabolism for the slices. If added, osmolality will increase by ~10 mOsm/kg.
- Equilibrate with 95% O2 and 5% CO2 before adding the CaCl2 and MgCl2.
- If you need to reduce/increase osmolality, reduce/increase the amount of sucrose to avoid modifying the other ion concentrations.
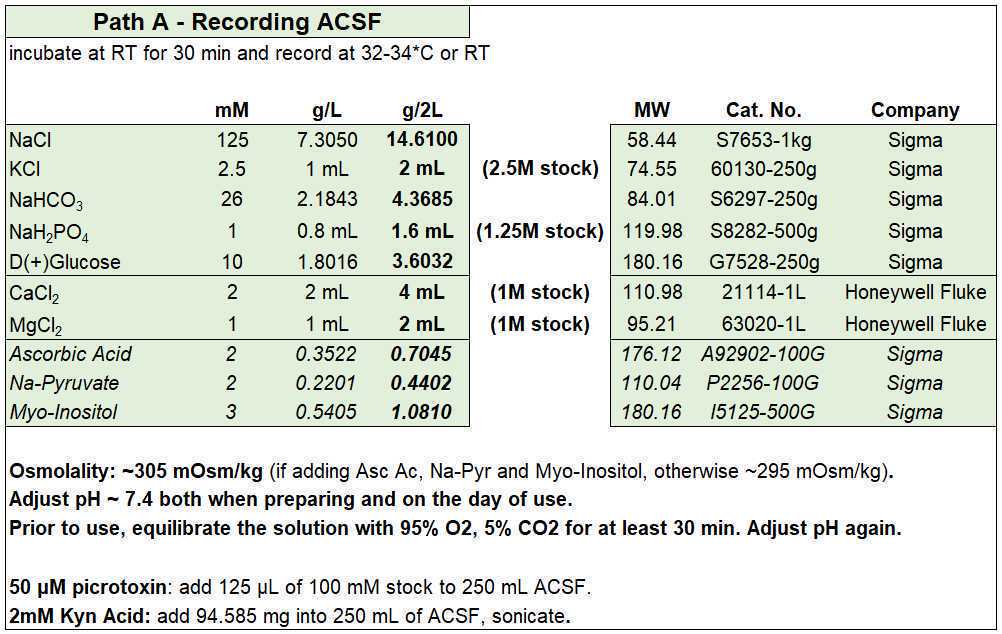
Recording ACSF
- For each experiment you will need ~150 mL for the second recovery chamber and ~250 mL for the recording (or more if you wash in specific compounds). Preparing 2 L is usually enough for the whole week.
- Recording ACSF (in mM): 125 NaCl, 2.5 KCl, 26 NaHCO3, 1 NaH2PO4, 10 D-glucose, 2 CaCl2, and 1 MgCl2 (equilibrated with 95% O2 and 5% CO2), with an osmolality of ~295 mOsm/kg.
- Optionally, you can add (in mM) 2 Ascorbic Acid, 2 Na-Pyruvate, and 3 Myo-Inositol. Ascorbic acid is a free radical scavenger, while Na-Pyruvate and Myo-Inositol are additional energy sources and entry points into cellular metabolism for the slices. If added, osmolality will increase by ~10 mOsm/kg.
- Equilibrate with 95% O2 and 5% CO2 before adding the CaCl2 and MgCl2.
[PATH B - Perfusion with ACSF under terminal isoflurane anaesthesia]
This version of the protocol follows the second of two alternative slicing methods, which briefly consists on intracardially perfusing the mouse with ice-cold slicing ACSF under terminal isoflurane anaesthesia, followed by decapitation, brain extraction, and slicing in ice-cold slicing ACSF, incubation in slicing ACSF for 10-12 min at 32°C, followed by incubation in holding ACSF for 1 h at RT, and finally transferral to recording chamber perfused with recording ACSF at 32-34°C or RT.
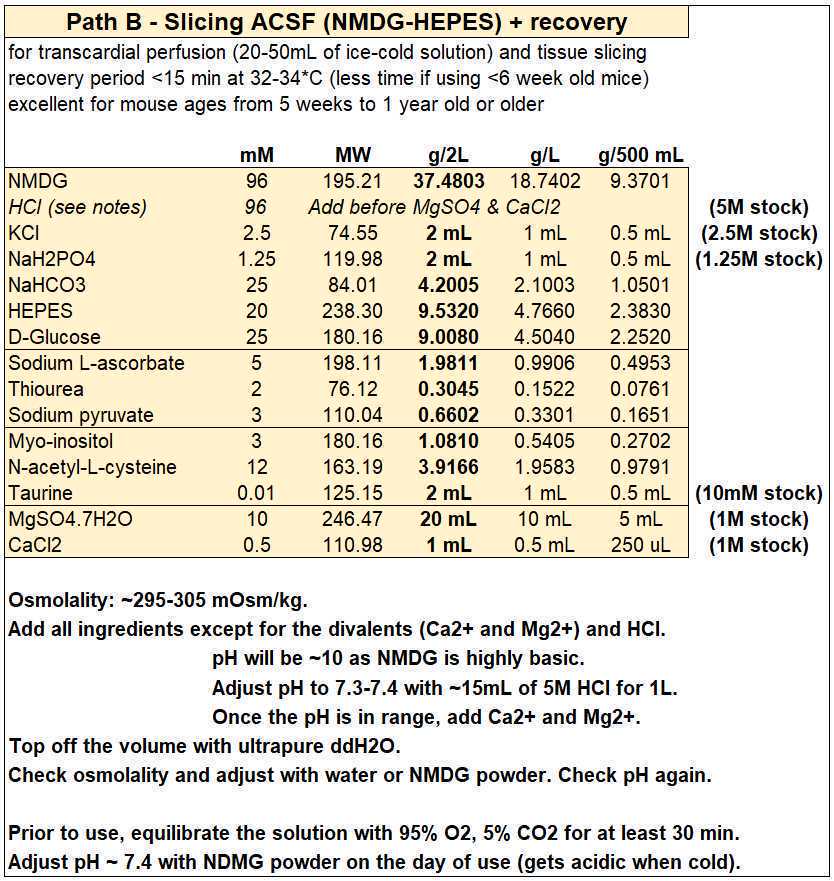
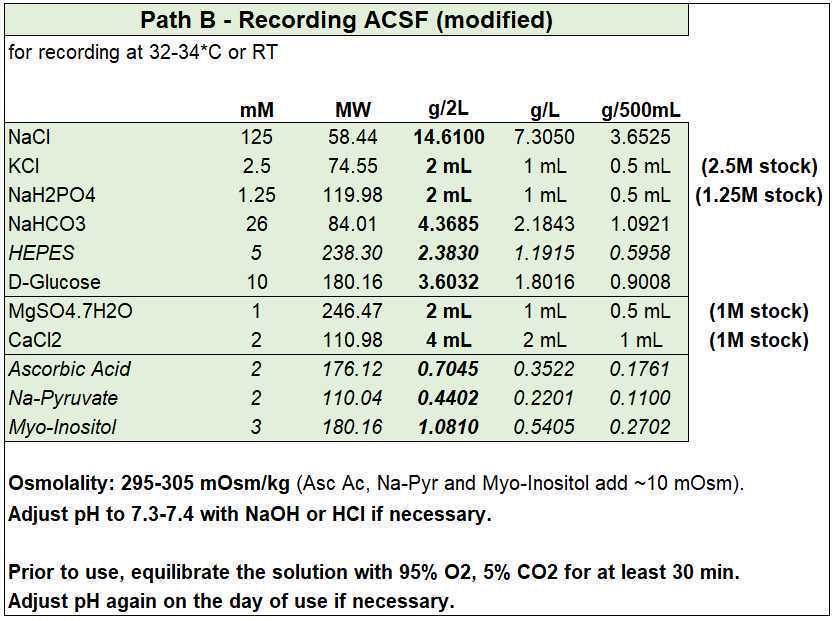
Slicing ACSF (NMDG-HEPES)
- For each experiment you will need ~500 mL (~150 mL for the first recovery chamber and ~350 mL to make the slush to be used for the intracardial perfusion and slicing steps), so by making 2 L on Monday you should have enough solution for four days of experiments.
- Slicing ACSF (in mM): 96 NMDG, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 20 HEPES, 25 D-glucose, 5 sodium L-ascorbate, 2 thiourea, 3 sodium pyruvate, 3 myo-inositol, 12 N-acetyl-L-cysteine, 0.01 taurine, 10 magnesium sulfate, 0.5 CaCl2, with an osmolality of ~300 mOsm/kg.
- Add HCl to bring pH in range before adding the CaCl2 and MgSO4.
- If you need to reduce/increase osmolality, reduce/increase the amount of NMDG (and adjust pH accordingly) to avoid modifying the other ion concentrations.
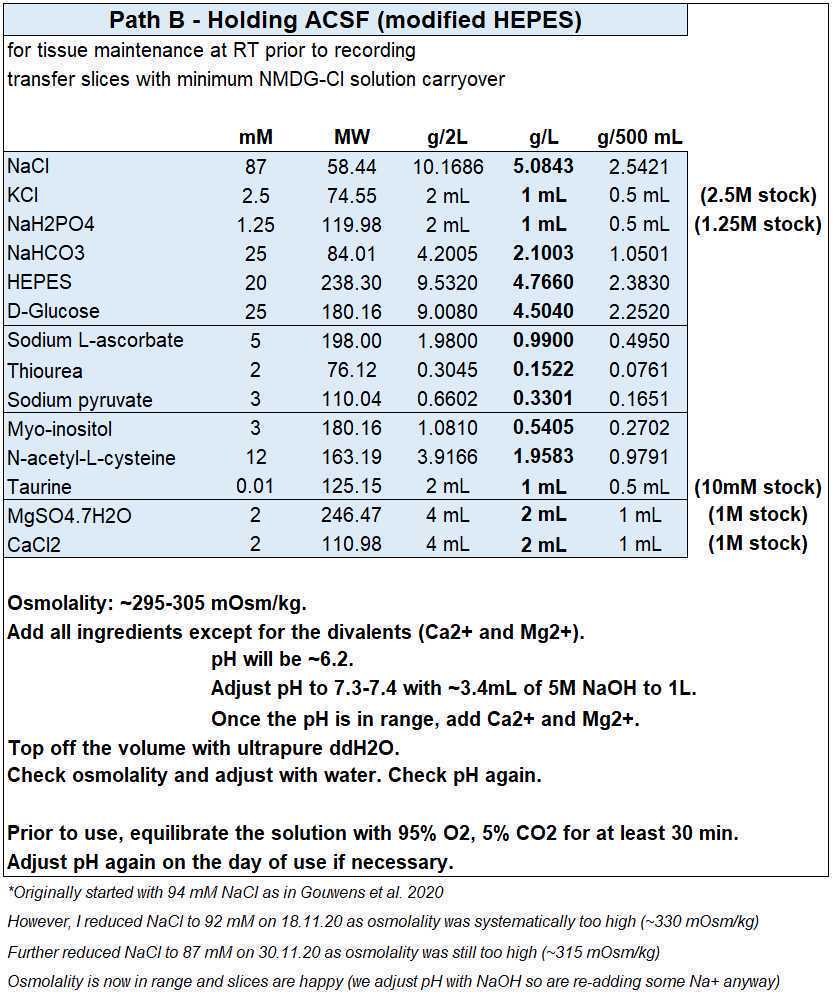
Holding ACSF (modified HEPES)
- For each experiment you will need ~150 mL for the second recovery chamber, so by making 1 L on Monday you should have solution in excess.
- Holding ACSF (in mM): 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 20 HEPES, 25 D-glucose, 5 sodium L-ascorbate, 2 thiourea, 3 sodium pyruvate, 3 myo-inositol, 12 N-acetyl-L-cysteine, 0.01 taurine, 2 magnesium sulfate, 2 CaCl2, with an osmolality of ~300 mOsm/kg.
- Add NaOH to bring pH in range before adding the CaCl2 and MgSO4.
Recording ACSF
The protocol provides the recipes for two alternative recording solutions, but you can further modify either of them to fulfill the specific needs of your tissue of interest. The second solution has been modified to contain HEPES (to increase pH buffering capacity in the physiological range), ascorbic acid (to reduce oxidative stress), and sodium pyruvate and myo-inositol (to provide additional entry points into cellular metabolism).
- For each experiment you will need ~250 mL for the recording (or more if you wash in specific compounds). Preparing 2 L is usually enough for the whole week.
[General considerations]
- Make sure your slicing area and tools are separate from any bench/area where PFA work is being conducted.
- Have a specific set of tools and glassware for slicing and slice electrophysiology.
- Mark all electrophysiology glassware (e.g. with a red dot) and make sure to wash it thoroughly with ddH20, independently from the rest of lab glassware.
- Sterilize the glassware used to prepare and store solutions and incubate slices by baking at 200°C for 2 h to minimise the risk of any bacterial growth. Do this at the end of each day after thoroughly washing with ddH2O to remove any salts residues.
- Always check the pH and osmolality of the stock solutions and keep a log for record keeping.
- Make sure to vigorously shake and mix the stock solutions before taking the amount you need for the day.
Preparation of tools, instruments, and solutions for the day
[Get solutions ready]
- Assemble two recovery chambers: for each chamber use a 150 mL glass beaker, a double-threaded plastic cap (or a 3D printed alternative as the one available here), some sterile dressing, and a 2 mL syringe from which the tip has been cut and a needle inserted at the bottom to make a support for the bubblestone. Ideally the chamber should be disassembled after every use to thoroughly clean with ddH2O and bake the glass beaker to sterilize it.
Depending on the path you follow, do:
- [PATH A - Decapitation under terminal isoflurane anaesthesia]: fill one recovery chamber with ~150 mL of slicing ACSF (sucrose) and the other with ~150 mL of recording ACSF. Place both beakers in the heating blocks (or water bath) so the solutions reach 35°C. Fill the large beaker with ~350 mL of slicing ACSF (sucrose) and leave on ice. Turn carbogen on and bubble all solutions for at least 20-30 min - this will ensure the pH is equilibrated and the solutions are oxygenated and at the right temperature.
- [PATH B - Perfusion with ACSF under terminal isoflurane anaesthesia]: fill one recovery chamber with slicing ASCF (NMDG-HEPES) and the other with holding ACSF (modified HEPES). Place both beakers in the heating blocks (or water bath) so the solutions reach 32°C. Fill the large beaker with ~350 mL of slicing ACSF (NMDG-HEPES) and leave on ice. Turn carbogen on and bubble both solutions for at least 20-30 min - this will ensure the pH is equilibrated and the solutions are oxygenated and at the right temperature. Importantly, remove the second recovery chamber with holding ACSF from the heating block before you start - this solution needs to be at RT and can be placed on the heating block to make sure it reaches RT quick enough (otherwise it may take longer to go from 4°C to RT).

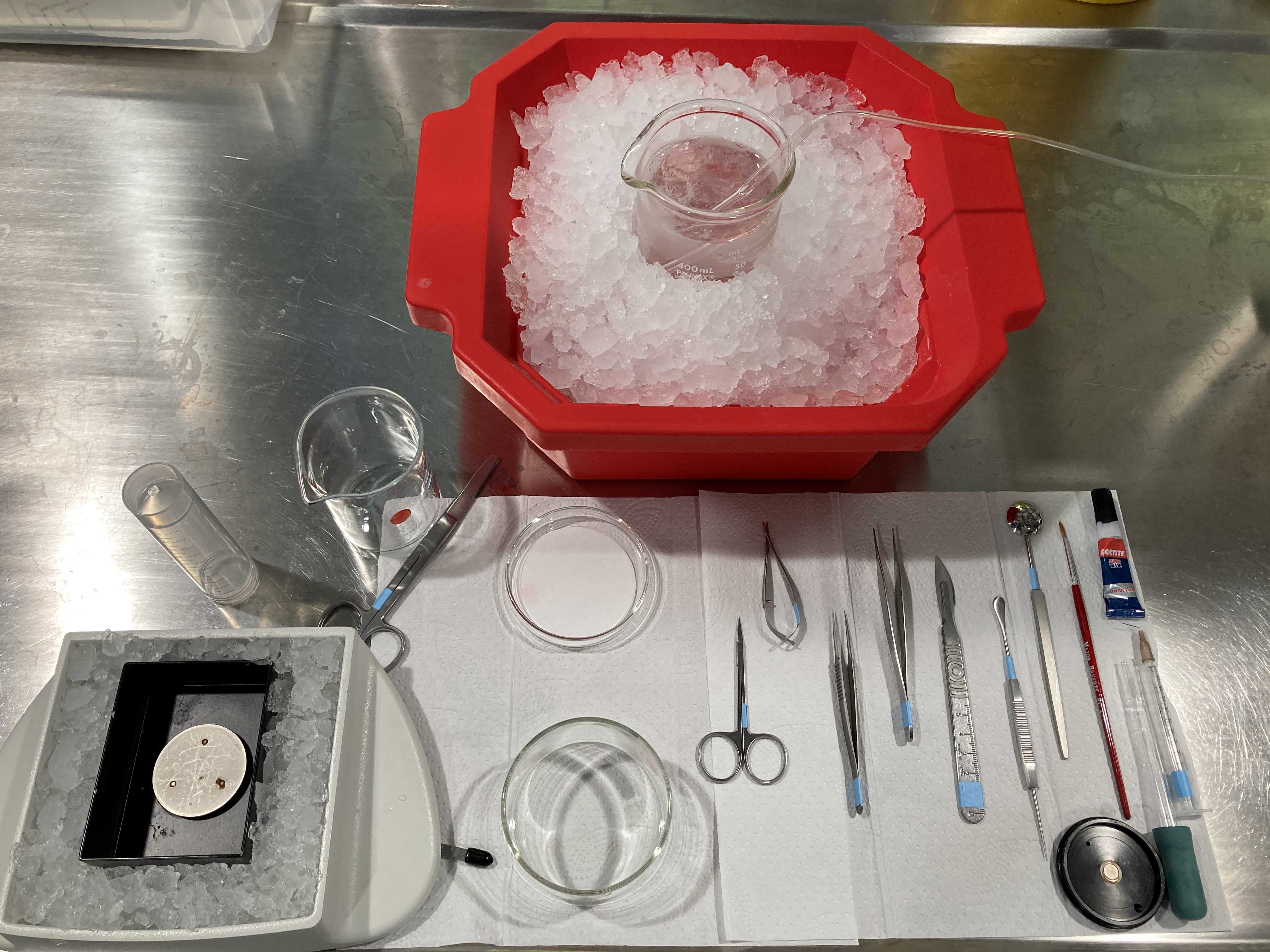
[Prepare tools for slicing - make sure to clean with ddH2O]
- Scissors for decapitation: Student Surgical Scissors Straight Sharp-Blunt (14.5 cm).
- Fine scissors for skin: Extra Fine Bonn Scissors Straight (8.5 cm).
- Small serrated scissors for skull: Vannas Tübingen Spring Scissors Straight Serrated (8.5 cm).
- Fine tweezers to detach meninges from brain: Dumont #5 Fine Forceps Biology Inox.
- Forceps to separate skull: Micro-Adson Forceps Serrated.
- Blade to cut frontal part of the brain and cerebellum: Scalpel Handle #4 and sterile blade.
- Spatula to remove brain from skull: Probe and Spatula (14 cm).
- Spoon to immerse brain in ACSF slush: Moria MC17 Perforated Spoon (20 mm tip diameter).
- Brush to move and reposition the slices: Major Brushes size 2.
- Superglue (Universal) to glue the brain on the magnetized block of the vibratome. Make sure it has not dried out.
- Syringe and needle to separate and mark cut slices from brain: 1 mL syringe with a stainless steel needle (e.g. brown - BD Microlance 3, 0.45x13 mm). Can be carefully washed and reused.
- Glass pipette to make a slice collector to pick and transport brain slices.
- Filter paper on a glass petri dish.
- Crystallizing dish without spout (150 mL) to perform the brain extraction (so that you can keep immersing the brain into cold solution at every step).
- Small 50 mL or medium 150 mL beaker to deposit the head after decapitation.
- Two medium 150 mL beakers for the recovery chambers.
- Big 400 mL beaker for the slicing ACSF.
- Falcon tube (50 mL).
- Ice pan full of ice.
- Double edge blades for vibratome (Personna).
- Cotton buds and Acetone to clean the blades before slicing.
- Tissue paper.
- Magnetized block from vibratome (where the brain will be glued before slicing).
[!] An additional thing you can try is to keep the tip of the tools on ice so they are as cold as possible during the brain extraction.
[Prepare slicing chamber]
- Assemble the support and the chamber and add some ice around the chamber (see bottom left of image below).
- Store in the freezer until needed to ensure it is as cold as possible.
- Leave the magnetized block out to prevent condensation when taking it out of the freezer as it can stop the glue/brain from sticking on to it (although it is possible to cover with paper to prevent that).
[Vibratome set up and blade alignment - Leica VT1200S]
- Get a Personna blade and carefully clean the grease (without bending it!): dip a cotton bud in acetone and use it to gently wipe away any dirt or grease on both sides of the blade. Then briefly rinse with 70% ethanol and then thoroughly clean with ddH20.
- Some people break the blade in half to use the second half the following day - we tend not to do that to minimise any unwanted bending of the blade.
- Place the blade on the vibratome and tighten. Do not lower it yet.
- Mount and connect the Vibrocheck. Then lower the blade to an horizontal position and follow the vibrocheck alignment procedure to align the blade. It is very important to do this with every new blade.
- Once it is aligned, keep it running for a while to make sure it doesn't change with time. Keep preparing the rest of things and stop it once everything else is ready.
- Exit the vibrocheck mode, move the blade holder up, and remove the vibrocheck and cable.
- The vibratome is now ready for use.

[Prepare slush with slicing solution]
In order to have the slicing solution as close to 0°C as possible, an Ice-Cream Maker can be used to prepare a slush.
- Make sure that the beaker with ~350mL of slicing ACSF has been bubbling for at least 20-30 min on ice.
- Before proceeding to make the slush, check the pH of the solution (pH is temperature dependent and will probably have changed). Adjust with either NaOH/NaHCO3 (if using the sucrose ACSF) or NMDG (if using the NMDG-HEPES ACSF). Once the pH is in range, proceed to the next step.
- Get the Ice-Cream Maker out of the freezer.
- Pour the ~350mL of bubbled slicing ACSF in and continue to bubble (see image below).
- Use a 50 mL falcon tube to scrape off the ice that generates in the walls and mix. Repeat until you have the perfect slush (you don't want too much ice nor too much liquid).
- Pour the slush of slicing ACSF back to the 400 mL beaker.
- Use a blender on the slush to avoid having sharp edges in the ice.
- Once smooth, put the beaker with the slush on ice and continue to bubble with carbogen until used.
- Clean the Ice-Cream Maker with ddH2O and dry it quickly with a tissue (if you are not quick enough some paper will stuck to the cold walls and will have to clean again). Put back in the freezer until next use.

[Hood and anaesthetic chamber]
- Place clean tissue paper on the floor of the anaesthetic chamber.
- Place a disposal bag in the large glass beaker (to dispose of the carcass).
- For PATH A - Decapitation under terminal isoflurane anaesthesia, make sure you bring the small beaker with the slush made of the slicing ACSF (sucrose) and the scissors for decapitation (image below).
- For PATH B - Perfusion with ACSF under terminal isoflurane anaesthesia , make sure you prepare a 20 mL syringe, four needles (to pin the paws), and the tray and silicon mat (where the animal will rest once anaesthetised). In addition, bring the small beaker with the slush made of the NMDG-HEPES ACSF and load the syringe with it. Get the scissors for decapitation, a pair of forceps, and medium-size scissors to carry out the dissection to reach the heart. To keep the tray as cold as possible, you can add ice around the silcon mat.
- Before you proceed to the next step and before you open the animal cage, make sure you switch the hood ventilation ON. Furthermore, switch the scavenging system and the O2 inflow ON before you open the anaesthetic flow.

[Electrophysiology rig]
- Fill a glass bottle with 250 mL of recording ACSF and cover with parafilm.
- Turn the carbogen ON and leave the recording ACSF bubbling until use so it reaches room temperature before you start recording.
Brain extraction
[Common for PATH A and PATH B - Get the experimental subject]
- Order animals at least a couple of days in advance - they have to be transferred from the breeding room to the satellite room (where the experimental animals are held).
- Try to plan so that they are between 6-10 weeks old on the day of the experiment. The older the animal, the most challenging the slicing and the more difficult to obtain good quality slices. If using transgenic lines, make sure you have enough breeding pairs to get enough animals of the appropriate genotype.
- When collecting the animal from the satellite room, follow your home institution guidelines regarding PPE: wear coat, gloves, hair net, and shoe covers before entering to the satellite room.
- Properly follow all protocols and record-keeping guidelines (labels, lab books, PyRat, etc.).
- Bring the cage to the slice electrophysiology area and place under the hood. Never open the cage with the ventilation OFF.
- Make sure absolutely everything you need is ready before proceeding to anaesthetise the animal. This includes regularly checking the weight of the scavenging filter (replace if limit is exceeded and properly dispose of the old one) as well as the levels of isoflurane in the anaesthetic system (top up if running low).
[PATH A - Decapitation under terminal isoflurane anaesthesia]
- Wear appropriate PPE: coat, mask, and gloves.
- Switch ON the ventilation hood. Only if ventilation is ON are you allowed to open the animal cage (otherwise allergens will be scattered everywhere).
- Put a disposal bag in the glass beaker if you haven't done so already.
- Pour some slush of slicing ACSF in the small 150 mL beaker. Get the beaker and the scissors for decapitation to the hood where the anaesthetic chamber is.
- Switch ON the oxygen flow from the pipe in the wall, and also turn ON the oxygen flow in the anaesthesia system to around 2 mL/min.
- Switch the scavenging system ON and double check a clean tissue paper is present on the floor of the anaesthetic chamber.
- Turn the valve behind the anaesthetic chamber so that it is on the "scavenging" position before opening the isoflurane flow (this system is there to protect you from any isoflurane leaks).
- Proceed to anaesthetize the mouse: open the animal cage and gently cup the mouse of your choice (we usually have ear marks to identify them when group housed) and transfer to the anaesthetic chamber. Avoid picking up by the tail as this leads to stress. Once the animal is in the anaesthetic chamber, switch the isoflurane pump ON by turning the wheel to 5 mL/min. Turn the valve behind the anaesthetic chamber to the "anaesthetise" position. While you wait for the isoflurane to have its effect, close the animal cage and move below the hood, so that the remaining animals can't see the rest of the procedure.
- Once the animal is under anaesthesia, open the chamber and check the reflexes by pinching the rear paw. If the anaesthesia levels are good, switch the isoflurane pump OFF and return the valve behind the anaesthetic chamber to "scavenging" position. Leave the scavenging system on for as long as you extract the brain.
- Take the animal out, hold over the beaker containing the slush of slicing ACSF and place the large scissors around the neck. With a swift cut, proceed to perform the decapitation, making sure the head falls inside the ice-cold and already bubbled slicing ACSF. Place the carcass in the bag of the large beaker.
- Quickly take the beaker to the vibratome area, making sure the head is completely immersed in the cold solution. The next steps are critical and must be done as quickly as possible - ideally the time between decapitation and immersing the extracted brain in the slush of slicing ACSF should be less than 1 minute.
[PATH B - Perfusion with ACSF under terminal isoflurane anaesthesia]
- Wear appropriate PPE: coat, mask, and gloves.
- Switch ON the ventilation hood. Only if ventilation is ON are you allowed to open the animal cage (otherwise allergens will be scattered everywhere).
- Put a disposal bag in the glass beaker if you haven't done so already.
- Pour some slush of slicing ACSF in the small 150 mL beaker. Get the beaker and the tools for dissection and decapitation (scissors for decapitation, scissors for skin, and forceps) to the hood where the anaesthetic chamber is.
- Load a 20 mL syringe with slush of slicing ACSF and mount a small brown needle on it. Press to squirt a bit of liquid and make sure there are no air bubbles in the syringe.
- Get the tray and the silicon mat together with four sterile small brown needles, which you will use to pin the animal. Place the anaesthetic mask on the silicon mat. Remove the cap from the needles and stick them on the silicon mat.
- Place the tools, syringe, and beaker on a piece of tissue paper next to the tray, ready to be used.
- Switch ON the oxygen flow from the pipe in the wall, and also turn ON the flow in the anaesthesia system to around 2 mL/min.
- Switch the scavenging system ON and double check a clean tissue paper is present on the floor of the anaesthetic chamber.
- Turn the valve behind the anaesthetic chamber so that it is on the "scavenging" position before opening the isoflurane flow (this system is there to protect you from any isoflurane leaks).
- Proceed to anaesthetize the mouse: open the animal cage and gently cup the mouse of your choice (we usually have ear marks to identify them when group housed) and transfer to the anaesthetic chamber. Avoid picking up by the tail as this leads to stress. Once the animal is in the anaesthetic chamber, switch the isoflurane pump ON by turning the wheel to 5 mL/min. Switch the valve behind the anaesthetic chamber to the "anaesthetise" position. While you wait for the isoflurane to have its effect, close the animal cage and move below the hood, so that the remaining animals can't see the rest of the procedure.
- Once the animal is under anaesthesia, open the chamber and check the reflexes by pinching the rear paw. If the anaesthesia levels are good, turn the valve to change the anaesthesia circuit from the chamber to the mask and transfer the mouse to the silicon mat. Carefully place the mask around the snout, making sure it stays into position. Switch the valve to change the scavenging circuit from the chamber to the mask and turn the valve behind the anaesthetic chamber from "anaesthetise" to "scavenging".
- With the mask well placed and the mouse still under anaesthesia, check the reflexes again. If the anaesthesia levels are still good, proceed to pin the animal to the silicon mat with one needle in each paw.
- Take the scissors with your dominant hand and the forceps with the other and proceed to make an incision on the skin at the level of the abdomen. Use the scissors to dissect up towards the rib cage until you see the diaphragm. Once you cut the diaphragm, the animal stops breeding properly and starts dying, so you need to be fast (otherwise perfusing will not be much of an improvement compared to decapitation). Carefully cut the diaphragm and cut around the rib cage until you see the heart. Carefully cut the right atrium - blood should start coming out. Quickly leave the scissors and get the syringe with the ice-cold slicing ACSF (squirt a bit of solution to ensure there are no bubbles in the tip). Use the forceps to position the heart and insert the needle in the left ventricle. Slowly empty the syringe without stopping, until all the solution has been used. If the perfusion works well, the liver should turn white and clean ACSF should be coming out towards the end.
- If you don't need the second hand to keep the syringe steady while perfusing, remove the mask from the snout and switch the isoflurane pump OFF (leaving the scavenging system on). Sometimes during the perfusion some ACSF comes out of the snout - removing the mask ensures the solution doesn't end up in the mask and makes a mess.
- Once the syringe is empty and the perfusion is done, remove the needles and take the big scissors. Carefully lift the mouse and hold over the beaker containing the remaining slush of slicing ACSF. Place the large scissors around the neck and, with a swift cut, proceed to perform the decapitation, making sure the head falls inside the ice-cold and already bubbled slicing ACSF. Place the carcass in the bag of the large beaker.
- If you haven't done so yet, switch the isoflurane pump OFF and leave the scavenging system on for as long as you extract the brain. After that, make sure you come back to change the valves back to the chamber circuit and leave the scavenging on for a bit longer. Remember to close the oxygen flow and the scavenger when you are done.
- Quickly take the beaker to the vibratome area, making sure the head is completely immersed in the cold solution. The next steps are critical and must be done as quickly as possible - ideally the time between decapitation and immersing the extracted brain in the slush of slicing ACSF should be less than 1 minute.
[Common for PATH A and PATH B - Brain extraction (<1 min)]
- Pour some slush from the 400 mL beaker on ice (where the slicing ACSF has been bubbling all this time) into the crystallizing dish. This is where you will keep immersing the head while doing the brain extraction, in order to keep it as cold as possible. Having a separate beaker for the extraction and decapitation also reduces the amount of blood carried over.
- Take the head from the small beaker and place in the crystallizing dish with clean slush.
- Hold the head to ensure the skull is stable by applying pressure to the snout (adopt a sort of pinching position with the thumb and index that surrounds the snout and with the maximal pressure on the phalanges - never apply pressure to the skull itself, as you will compress and damage the brain). Have your middle finger beneath it as a support. Importantly, you should ensure you practice this in advance, having a good and stable grip will be critical to extract the brain as quickly and safely as possible.
- Use the medium size scissors to cut through the skin along the midline and expose the skull. Start at the back of the neck and make your way until the snout. Fully immerse the head in the ice-cold slush slicing ACSF.
- With the brain still immersed in the slush, switch to the tiny serrated scissors. Start to very carefully cut through the bone from the foramen magnum in the following sequence.
- (1) The first two cuts will be to the sides, from the foramen magnum around the temporal bone until the zygomatic bone or the ear canal (surrounding the cerebellum). This two cuts (one to the right and one to the left) are very important as will reach the insertion of most meninges: making sure these are cut will prevent they slash through the brain and separate the cerebellum and inferior colliculus from the superior colliculus and periaqueductal gray.
- (2) Next, once again insert the tiny serrated scissors in the foramen magnum and proceed to cut the skull along the midline suture from caudal to rostral until you reach the snout. Make sure you cut "up" when you advance with the scissors (never "down" towards the brain) to avoid pressing the bone on the tissue. Fully immerse the head in the ice-cold slush slicing ACSF.
- (3) Finally, make two tiny cuts from the midline to the laterals at the level of the olfactory bulb. Fully immerse the head in the ice-cold slush slicing ACSF.
- N.B : although you must be fast, it is also critical to be very careful and to keep mechanical stress to the minimum - the midbrain is incredibly sensitive. In addition, as already mentioned above, always make the scissor cuts in the upper direction, so that instead of applying pressure to the skull you are lifting it away from the brain. In many cases, insufficient slice quality is due to problems at this very early stage.
- With the brain still immersed in the slush, switch to the fine Dumont forceps. Very carefully lift each half of the skull and check if the dura mater is still stuck to the brain or it instead lifts with the bone. If the dura is still there, make sure you carefully detach it by inserting the tip of the forceps right below it and moving the tip across the bone (from front to back). Do the same for both sides and make sure the meninges are out of the picture before proceeding. Fully immerse the head in the ice-cold slush slicing ACSF. This is a critical step, if not done the remaining meninges can cut the entire brain when you lift the skull in the next step.
- With the brain still immersed in the slush, switch to the thick serrated forceps. Remove the skull by lifting the cut bone with the forceps while moving the hand that holds the head in the opposite direction. Do the same for the other side. Fully immerse the head in the ice-cold slush slicing ACSF.
- With the brain still immersed in the slush, take the blade. Make sure you are holding the head as flat as possible. Make one cut at the level of the cerebellum and one in the front to remove a good chunk of the frontal lobe. It is very important that this second cut is as straight as possible as this is the surface the brain will stand on when we glue it.
- With the brain still immersed in the slush, grab the spatula and carefully scoop the cut parts out. Then carefully separate the useful part of the brain from the skull and gently transfer (do NOT drop) to the 400 mL beaker with the remaining slush of slicing ACSF (which has been bubbling on ice until now). Use the perforated spoon to carefully scoop a pocket in the slush so that the brain falls and remains completely submerged in it. Make sure the bubbling is not too close to the brain.
- Now the hardest bit is done and you can breath. This should have taken less than a minute. You can leave the brain to recover for a minute or so in the cold solution, this will ensure to slow down the metabolism and reduce excitotoxicity.
- In the meantime, bring the now empty skull back to the hood and put in the plastic bag together with the carcass. Properly dispose of the carcass. Turn off the oxygen from the wall and watch the rubber that indicates the flow rate fall to zero. Make sure the isoflurane is off. Then close the circuit from the anaesthetic chamber and switch off the scavenging system. Clean the area with ddH2O from any blood or ACSF and wipe with 70% ethanol. Switch the hood ventilation off. Bring the beaker and scissors to the sink. You can finish cleaning up later.
- Get the slicing chamber from the freezer. Make sure the magnetized block is dry and the glue hasn't hardened.
- Use the perforated spoon to carefully lift the brain out of the beaker. Take extra care not to push it against the glass wall. Gently place on the filter paper in the petri dish. Add enough slush ACSF to cover it. Use the brush to carefully remove any slush that has stuck to the brain, so that it is free. Re-position the brain so that it sits on the cut you made in the frontal part of the brain (i.e. the cerebellum is facing up to you).
- Put glue on the magnetized block. Make sure there is enough to glue the brain, but not so much the brain swims on it.
- With the spatula on your dominant hand and the brush on the other one, carefully load the brain on the spatula so that the frontal side faces down (i.e. the bit where the olfactory bulb would have been is in contact with the spatula, and the cerebellum is facing up towards you). Next, carefully lift the brain and gently touch a clean tissue paper to dry out any excess liquid (this will ensure the tissue sticks to the glue). Place the brain on the glue and use the brush to gently press the cortex down to ensure it properly sticks to the magnetized block (only do that if you don't care about the cortex). N.B : be very gentle when rotating the brain and moving it to the block. Do not hit the magnetized block against the table and avoid any unnecessary mechanical stress to the tissue.
- Rapidly use the glass pipette to pour some ice-cold slicing ACSF from the petri dish over the brain - this will both keep the brain cold and harden the glue.
- Carefully mount the magnetized block on the slicing chamber and proceed to fill the chamber with ice-cold slicing ACSF. Use the perforated spoon to protect the brain, so that the slush doesn't fall on it too hard (minimise mechanical stress).
- Place the slicing chamber on the vibratome and lock it.
Slicing
[OPTION 1 - Slicing with a Leica VT1200S]
-
Mount the slicing chamber and fix the tiny bubbler to the slicing chamber and immerse to constantly equilibrate the solution with carbogen during slicing.
-
With the brush, move the slush away from the brain.
-
Lower the blade to horizontal position.
-
Bring the slicing chamber up by pressing "UP" until the top of the brain is at the level of the blade. Press the button to move the blade closer to the brain.
-
Set the setting to "Manual" and see below for a note on how to slice in this mode.
NoteIn the "Manual" mode of the Leica, slice as follows: press "RUN/STOP" to start slicing, set the desired speed with the top wheel, press "RUN/STOP" to stop slicing, use the bottom wheel to bring the slicing chamber "DOWN" for 100 microns (i.e. away from the blade, so that you are no longer touching/pushing the brain down and you don't press on it when collecting the slice and retracting the blade), press the button to move the blade back (i.e. away from the brain) until completely out, use the bottom wheel to bring the slicing chamber "UP" for 350 microns (i.e. set the desired slice thickness remembering you first moved 100 microns in the opposite direction, so the thickness of this slice will be 250 microns), press "RUN/STOP" to resume slicing. Repeat until done. -
At the beginning, set the slicing speed to 0.10 mm/s and the amplitude to 1 mm. In the interest of time, slice thicker sections (500 to 900 microns) at this speed until you reach PAG (or the area of interest).
-
Once you start to see the Aqueduct, you are getting close to PAG. Set slice thickness to 250 microns and speed to 0.05 mm/s (see note above for details, keep the amplitude at 1 mm).
-
While slicing, and as long as you don't need it, you can use the brush to carefully get rid of the cortex.
-
Once the blade has surpassed PAG, stop the slicing and go 100 microns down (see note above for details).
-
Use the needle to carefully cut the slice to separate it from the brain, making sure you don't touch the sharp end of the blade and without excessively pressing on the blade. Make a cut at the right side, so you can orient the slice in the scope and know what is right and left in the images from the recorded cells.
-
Using the glass pipette, collect the slice and transfer to the first recovery chamber. According to Bischofberger et al. (2006) it is better to place the slide upside down from how it was sliced, as the tissue is healthier there.
-
Proceed to obtain the next slice. Repeat the cycle until done. Preparing 250 microns thick slices will give you 4-5 usable slices containing PAG.
-
Try to place the slices on the recovery chamber following the order of slicing. This will allow you to easily locate the coordinates of each slice and keep track of which are the freshest slices.
-
Given that the slicing speed is very low, you can start cleaning the tools and glassware you have finished using. Rinse everything with ddH2O and dry. Put back where it belongs. Leave the glassware in the drying rack until baking.
-
Once finished, proceed to clean the vibratome. Carefully remove the blade and thoroughly rinse the blade holder with ddH2O to remove any salts (make sure the slicing chamber is still underneath so it collects the water). Remove the slicing chamber and take the magnetic block with the rest of the brain out - use the blade to scrape off the glue and the rest of the tissue. Dispose of the blade in a sharps bin. Disassemble the slicing chamber and thoroughly wash with ddH2O. Leave to dry.
-
Using a piece of paper, carefully dry the blade holder and the vibratome. Place a piece of filter paper where the blade slots in, so it absorbes any water left. Lower the blade holder and switch vibratome off.

[OPTION 2 - Slicing with Campden Ci7000smz-2]
- Switch ON (at the back). The advantage of using this vibratome is that you do not need to calibrate or align the blade each time, as it uses a ceramic blade that only needs calibrating when you first mount it or replace with a new one. It is also possible to use ceramic blades on the Leica.
- Mount the slicing chamber, careful not to touch the blade.
- Immerse the tiny bubbler in the solution of the slicing chamber and fix to constantly bubble the solution with carbogen.
- Select slicing program (you have created it in advance).
- With the brush, move the slush away from the brain.
- Press "Load Bath" for a long time - the chamber will go up.
- Press "Height UP". Then press "Advance", followed by "Slice ON" - this will approach the blade to the brain. Stop when close but before touching the brain. This point will be recorded and the blade will go back there whenever you press "Return".
- The thickness of the slice is predetermined in the program, in our case 250 microns.
- Start slicing by pressing "Slice ON - Slice OFF - Return" until you reach the PAG or area of interest. Repeat as many times as needed (each cycle goes down 250 microns).
- Once in the area of interest, press "Slice ON" and adjust speed to 0.04-0.06. While slicing, and as long as you don't need it, you can use the brush to carefully get rid of the cortex.
- Once the blade has surpassed PAG, press "Slice OFF". Use the needle to carefully cut the slice to separate it from the brain, making sure you don't touch the sharp end of the blade and without excessively pressing on the blade. Make a cut on the right hemisphere, so you can orient the slice in the scope and know what is right and left in the images from the recorded cells.
- Using the glass pipette, collect the slice and transfer to the first recovery chamber. According to Bischofberger et al. (2006) it is better to place the slide upside down from how it was sliced, as the tissue is healthier there.
- Try to place the slices on the recovery chamber following the order of slicing. This will allow you to easily locate the coordinates of each slice and keep track of which are the freshest slices.
- Press "Return": this takes the blade back to the starting point, and lowers it 250 microns for the next slice.
- Repeat until done. Preparing 250 microns thick slices will give you 4-5 usable slices containing PAG.
- Once done, press "Load Bath" for a long time - the chamber will go down.
- Thoroughly rinse the blade with ddH2O.
- Remove the slicing chamber and thoroughly clean with ddH2O. Leave to dry.
- Using a piece of paper, carefully dry the blade from the back (never touch the sharp edge).
- Place safety cover on the blade (red bit with magnet).
- Switch OFF.
[Common considerations - Clean up and record keeping]
- Rinse the bubblestones you have used with ddH2O and immerse in a beaker with ddH2O and leave bubbling until the end of the incubation so it gets rid of any salt residues.
- Make sure you properly clean all the tools and instruments you used. Carefully dry and put them back where they belong.
- Squirt some ddH2O on the bench where you have done the brain extraction and around the vibratome, and wherever slicing ACSF may have fallen. Wipe surfaces and dry. Leave everything as you found it (or better).
- At the end of the day, bake all glassware at 200°C for 2 h to sterilize, so that everything is ready for the next morning.
- Update your logs accordingly with the animal used and the procedure carried out.
- Return the animal cage to the satellite room.
Incubation
[PATH A - Decapitation under terminal isoflurane anaesthesia]
In this path, the first recovery chamber contains slicing ACSF (sucrose) and the second recovery chamber contains recording ACSF. Both have been heated to 35°C and equilibrated with carbogen until now. Any bubbles stuck to the net have been removed prior to transferring the slices. Once all the slices have been transferred to the first recovery chamber, proceed to the incubation:
- Incubate slices for 30 min in slicing ACSF (sucrose) at 35°C, constantly equilibrated with 95% O2 and 5% CO2.
- If the slices express fluorescence or channelrhodopsin, cover with aluminum foil to protect from ambient light.
- After 30 min, carefully remove the second recovery chamber containing recording ACSF from the heating block and place on the bench at RT. Use the glass pipette to transfer each slice from the first to the second recovery chamber (from slicing to recording ACSF). Make sure to minimize any solution carryover when transferring the slices (you want as little sucrose and magnesium as possible added to the new solution).
- Once all the slices have been transferred to the second recovery chamber, let equilibrate to RT for 30 more minutes before recording. Constantly equilibrate the solution with 95% O2 and 5% CO2.
[PATH B - Perfusion with ACSF under terminal isoflurane anaesthesia]
In this path, the first recovery chamber contains slicing ACSF (NMDG-HEPES) heated to 32°C and constantly equilibrated with carbogen. The second recovery chamber contains holding ACSF (modified HEPES) and has been heated to 32°C until slicing and then removed from the heating block and placed on the bench at RT, constantly equilibrated with 95% O2 and 5% CO2.
In this case, the incubation procedure will be slightly different, and the incubation of each slice will be timed independently.
- Incubate each slice for 10-12 min in slicing ACSF (NMDG-HEPES) at 32°C, constantly equilibrated with 95% O2 and 5% CO2.
- After the 10-12 min, carefully transfer each slice to the second recovery chamber containing holding ACSF (modified HEPES), ensuring minimal solution carryover. Incubate for 1 h at RT before recording, constantly equilibrating the solution with 95% O2 and 5% CO2.
- If the slices express fluorescence or channelrhodopsin, cover with aluminum foil.
[Common considerations]
After the incubation period is over, use the glass pipette to collect the slice you will start recording from and transfer to the recording chamber in your rig. Place a platinum ring with equidistant nylon threads on top to stably hold the slice in place.
- It is important to make sure any storage/slicing solution is washed out before you start your recordings. The slice must be perfused for a sufficiently long time (at least 10 min for a flow rate of 3–5 ml/min) with recording ACSF before starting your experiment. This is important because the different concentrations in the holding ACSF can cause sealing problems and affect both excitability and synaptic transmission.
Clean up
Tools : thoroughly wash all tools and glassware with ddH2O, making sure you get rid of any blood or salts residues. If any of the tools used during brain extraction have been in contact with glue, use a blade to scrape it off before cleaning and drying the tool.* Consumables: properly dispose of any consumables or PPE that have been contaminated by blood or organic tissue using the appropriate bin. Furthermore, if you finish any items (filter paper, syringes, glass pipettes, needles, blades, salts, water, gloves, plastic bags, tissue paper, falcon tubes, glue, etc.) make sure to replenish the stock.
- Properly dispose of any sharps : needles, blades, and broken glass go to a sharps bin.
- Properly dispose of the carcass according to the guidelines and protocols in place at home institution.
- Clean the bubblestones: rinse with ddH2O and then transfer to a beaker with fresh ddH2O. Immerse them and leave them bubble for a while. At the end of the day, transfer to a separate beaker with a piece of paper at the bottom and leave to dry. Switch carbogen off.
- Glassware: thoroughly wash all glassware with ddH2O to remove any salt residues from the ACSF solutions. Leave in the rack to dry until the end of the experiment. Then proceed to sterilize by baking at 200°C for 2h, to make sure bacteria can't grow there. If the oven has a timer, you can do this at the end of the day so that it has time to cool down and all glassware is sterile and ready to be used the next morning.
- Vibratome: carefully remove the blade and thoroughly rinse the blade holder with ddH2O to remove any salts (make sure the slicing chamber is still underneath so it collects the water). Remove the slicing chamber and take the magnetic block with the rest of the brain out - use the blade to scrape off the glue and the rest of the tissue. Dispose of the blade in a sharps bin. Disassemble the slicing chamber and thoroughly wash with ddH2O. Leave to dry. Using a piece of paper, carefully dry the blade holder and the vibratome. Place a piece of filter paper where the blade slots in, so it absorbes any water left. Lower the blade holder and switch vibratome off.
- Bench surfaces: spray some ddH2O on the bench where you have done the brain extraction and the area around the vibratome, and wherever slicing ACSF may have fallen. Wipe surfaces and dry. Leave everything as you found it (or better).
Troubleshooting slice quality problems
[Slicing]
A critical question to answer when troubleshooting slice quality is: do the slices look good when you just slice them? What about after the incubation is over? Do they look good immediately after you place them in the recording chamber, or do they already look dead then? The answers to these questions will direct you to which of the steps is more likely to be the problem.
- If the slices look already bad right after slicing, you can start by checking the slicing solutions (try making a new batch), the vibratome (perhaps it needs servicing), or your brain extraction technique (you may need to do it faster or more careful, or you may need to try intracardially perfusing the animal with slicing ACSF).
- If the slices look good right after slicing, but bad after incubation, you probably have an issue with either the incubation time, temperature, or solution. It could also be that the recovery chamber is contaminated or the solution is not sufficiently oxygenated.
- Finally, if the slices look perfect right after the incubation, but degrade quickly once you put them in the recording chamber, you may be having issues with the recording ACSF, the temperature in the bath (malfunctioning heater), the oxygenation of the solution, or excitotoxicity of the tissue (recording at RT should reduce this).
Other general things to try are:
- When troubleshooting, try to start with younger animals first. Tissue from young animals is usually more forgiving to the procedure and will help you find what the major problem is.
- Keep temperature low (0-4°C) and dissect as fast and as careful as you can - when extracting the brain, make sure to immerse the head and brain in ice-cold solution at each step to keep as cold as possible.
- Intracardially perfuse the animals with ice-cold slicing ACSF before extracting the brain. This is the best way to clear the blood out of the brain and rapidly slow metabolic activity by replacing it with the solution of your choice (e.g. low sodium and calcium and high magnesium and NMDG). Again, this may work better or worse depending on the brain area and age of the animals you work on (it seems to make no difference in younger animals).
- The exact duration of the recovery period is critical for obtaining the optimal tissue preservation. For example, it has been shown that an excessively long recovery in NMDG ACSF will result in excellent morphological preservation but poor functional recovery, whereas a shorter recovery in NMDG ACSF will result in more complete functional recovery but poorer morphological preservation. Try to play with incubation times to find the sweet spot for your particular brain area.
- Regardless of whether you perform your recordings at room or physiological temperature, the incubation step at RT is critical as it allows the slices to recover from spine sprouting, which occurs when slicing (see Edwards, et al. (1989).
- Check the slice quality of a colleague using the same equipment and solutions than you. This can help you rule out problems with carbogen, water, or slicing machine. However, beware of differences between brain areas (the same procedure can give an excellent quality for visual cortex and kill all your cells in the periaqueductal gray).
[Example of a slice troubleshooting cycle]
After a series of bad experiments, we followed this sequence of steps to solve our slice quality issues:
- We moved to bottled ultrapure water to make solutions (a previous troubleshooting cycle ended when discovering a problem with the water source we were using), and cleaned/calibrated the osmometer.
- We ordered new glassware and thoroughly washed it, and we started baking it to sterilize it.
- We replaced all bubblestones and tubing, renewed all salts, made new stocks (e.g. 2.5M KCl and 1.25M NaH2PO4), made new recovery and recording chambers, flushed tubing with EtOH and ddH2O, and thoroughly washed all glassware.
- We tried to get some sanity checks done: test water source, carbogen outlets, and service the vibratome. We asked to use a (new) vibratome from a neighouring lab until ours could be serviced.
- We started intracardially perfusing our animals with ACSF, as most people agrees this helps keep the tissue in better condition (especially in adult animals), and tried more protective solutions (NMDG and Choline based).
- We watched several other people slice and compared their approach and solutions. We introduced further recommendations such as testing and adjusting the pH right before use (solutions turned acidic when cold).
- We compared different approaches by slicing pairs of young animals and changing one thing at a time: one perfused vs. one decapitated, one with a longer incubation vs. one with a shorter, one sliced with a vibratome vs. one with a different machine, one with our usual solution vs. one with a new recipe, one with the usual blade vs. one with a new ceramic blade, etc.
- After a series of rounds of testing, and always sticking to the best option of each comparison, we found we obtained the best improvements when fixing the vibratome (it turned out it had a malfunctioning piece), changing the solutions (NMDG and HEPES holding gave significantly better results), and intracardially perfusing the animals.
[Literature and resources used]
- Conversations with Nicolas Wanaverbecq, Annalisa Scimemi, Vanessa Stempel, Yaara Lefler, Yu Lin Tan, Simon Weiler, Sara Mederos, Hedi Young.
- Brain Slice Methods website by Jonathan Ting [link].
- Allen Institute Technical White Papers [link].
- NeuroWire blogs by Jonathan Ting [link] and Nour Al-muhtasib [link].
- Gouwens, Sorensen, et al. (2020) [https://doi.org/10.1101/2020.02.03.932244].
- McCauley, Petroccione, et al. (2020) [https://doi.org/10.1016/j.celrep.2020.108255].
- Wilson, Dohare, et al. (2020) [https://doi.org/10.1101/2020.05.22.109462].
- Dorst, et al. (2020) [https://doi.org/10.1038/s41467-020-18882-y].
- Gouwens, Sorensen, Berg, et al. (2019) [https://doi.org/10.1038/s41593-019-0417-0].
- Mandelbaum, et al. (2019) [https://doi.org/10.1016/j.neuron.2019.02.035].
- Bellini, Fleming, De, et al. (2018) [https://doi.org/10.1523/JNEUROSCI.1906-17.2017].
- Ting, Lee, et al. (2018) [https://dx.doi.org/10.3791/53825].
- Ting, et al. (2014) [https://doi.org/10.1007/978-1-4939-1096-0_14].
- Edwards, et al. (1989) [https://doi.org/10.1007/BF00580998].
[Vibratome]
It is critical that the machine you use to prepare slices is of a high quality and in pristine condition.
- Make sure the vibratome you use is routinely serviced.
- Always calibrate the z displacement of the blade to make sure it is not inflicting unwanted mechanical damage on your slices.
- Try using ceramic blades, but beware they may or may not improve your slice quality depending on the brain region you study. Replace the blade if old or blunted.
- Change the angle or the speed at which you slice - again, the usefulness of this may depend on the brain region you work on.
- If you are not sure your vibratome is the problem, try to find a neighouring lab which currently gets good slices and try slicing in their machine to rule out problems with yours.
[Solutions]
- If working with adult animals, switch to solutions optimised for adult brain tissues such as NMDG- or Choline-based ACSF, where NMDG-Cl or Choline-Cl replaces NaCl. Together with a low temperature, this will help reduce excitotoxicity.
- In the Brain Slice Methods website, Jonathan Ting argues that neuronal membranes appear heavily damaged/dimpled when slices are exposed to high sucrose ACSF during recovery at elevated temperature of 32-34°C. This may suggest that sucrose becomes cell permeable, or alternatively that its permeability-blocking role is compromised at higher temperatures. It is worth comparing the results between the high sucrose slicing solution and the NMDG/Choline solution in your hands.
- Adding 20 mM HEPES can effectively provide stronger pH buffering in the physiological range and prevent excessive oedema.
- A low Ca / high Mg ratio in the slicing solution will ensure that neural excitability is very low. Reducing the calcium driving force ensures there is less entry of calcium and therefore less activity and neurotransmission, and increasing magnesium keeps NMDA receptors blocked.
- You can also try adding supplements for energy supply and antioxidant function. Myo-inositol and Na-pyruvate provide additional entry points into cellular metabolism, whereas ascorbic acid, thiourea, and N-acetyl-L-cysteine (a cell-permeable glutathione precursor) help reduce oxidative stress.
- As mentioned above, it is critical to use purified water that is free of contaminants such as trace metals. Impurities like these are often found in distilled water sources and cause damage to the slices and can even convert anti-oxidants such as ascorbate into pro-oxidants.
- Make sure your solutions are fresh, ideally less than 24h but certainly not older than a week.
- Minimise carryover when transferring slices between solutions: an extreme way of doing this would be having an intermediate beaker (so you transfer the slice to the first beaker, and then from there to the second) so that any carryover is diluted.
- To rule out problems with your solutions, try slicing with solutions from a colleague working in a different lab. Alternatively, borrow one of their slices to check they are equally happy in your rig and theirs.
[Water]
Another usual suspect is the water you use to prepare solutions. It is possible that the water source has become contaminated or the filters saturated.
- If in doubt, purchase commercially available ultrapure ddH2O and make fresh solutions with it. One of the first things to rule out should always be the batch of ACSF you used.
- Make sure all the solutions and stocks (including 1M stocks of certain salts) are made with ultrapure water.
- It is advisable to regularly send samples from your water system to analyse to compare quality to UltraPure (e.g. Severn Biotech) and Molecular Grade Sterile Water (e.g. Corning) and to check for bacterial contamination.
- Ensure proper maintenance of the water purification system: replace filters, run a cleaning and sterilization cycle, replace UV lamps, etc.
[Salts]
It is possible that the salts and chemicals you use to prepare the solutions have gone bad or you received a contaminated batch.
- Some salts (such as Na-Pyruvate) can go bad quicker than others. Keep track of opening date and replace if older than 3-6 months.
- If nothing else works, replace all salts for new ones and make fresh solutions.
[Glassware]
Another important point is to avoid bacterial contamination and salt build up in your glassware.
- Thoroughly clean the glassware and tools you use to prepare solutions, with special emphasis on the recovery chambers.
- Have a separate set of glassware specifically dedicated to your slice work.
- Sterilize the glassware by baking it for 2 h at 200°C before every use.
- You can use 0.1M nitric acid to help get rid of any salt build up on the glass, but make sure you thoroughly clean with ultrapure ddH2O afterwards.
[Carbogen]
It is also possible that the solutions and slices are not sufficiently oxygenated.
- Set the bubbling really high whenever possible to ensure you oxygenate the tissue as much as possible, and always equilibrate solutions for 20-30 min prior to use.
- Test the carbogen outlets to ensure the mixture has the right concentration (95% O2 and 5% CO2).
- If using tanks, make sure it is not empty or you did not get a bad batch.
[Osmolality, pH, and temperature]
- Make sure the osmometer and pH meter are properly calibrated and clean.
- Always check the osmolality and pH of your solutions. Importantly, both values may change after 30 minutes of bubbling or depending on the temperature. Cover with parafilm to minimise evaporation when bubbling.
- If you need to reduce osmolality of the slicing solution, try to change the amount of sucrose or NMDG/Choline, so that you don't modify the other ion concentration.
- pH is temperature dependent and slicing ACSF can turn acidic after bubbling for 30 min on ice (0-4°C). Do check if that is the case and correct if necessary so it is as close to 7.3-7.4 as possible. A low pH will certainly have an effect on the quality of the slices.
- Always use an external thermometer to double-check the temperature in the bath, recovery chamber, and recording chamber. Make sure it is what you are expecting.
- Surround the slicing chamber with ice and keep in the freezer until use to keep the temperature as low as possible.
[PFA contamination]
- Always rule out an accidental PFA contamination in your tubing, bench area, glassware, tools. Make sure you work with tools and on a lab area dedicated to slice work whenever possible, including for perfusing the animals and extracting the brain.
Patch-Clamp rig maintanance
[Optics]
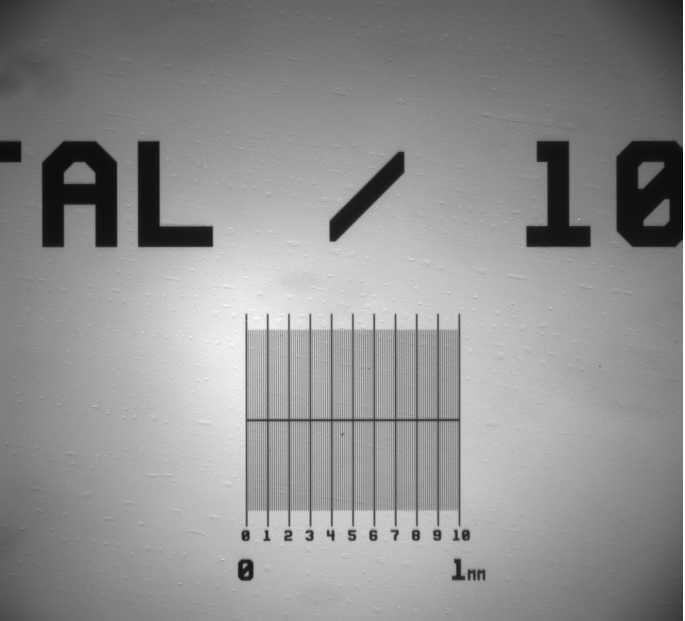
- Camera calibration: use a microruler to calibrate the camera on your rig. You want to take a picture and measure the distance in pixels between a section of the microruler. Use the resolution of the camera and the microruler to obtain the pixel to micron relationship in order to be able to add the correct scale bar to any pictures you take. Make sure you do this for all the objectives you will use.
- Kohler illumination : make sure you focus and center the condenser to obtain Kohler illumination. Do this on the high magnification objective (the one you will use to select cells) so that you have the optimum contrast and resolution when looking for cells. There are plenty of guides on how to do this on-line, including these from MicroscopyU, Zeiss, Olympus, Scientifica, or Microscopist.
- References to help you choose between oblique illumination, differential inference contrast illumination, and infrared light source (770 nm or 900 nm): [Oblique - MicroscopyU], [Oblique - Scientifica], [DIC - MicroscopyU], [DIC - Scientifica], [IR - BrainSlice Methods].
[Bubblestones]
- If you notice mould starts to grow on the tip of the bubblestone, you can try to clean it by leaving it immersed in H2O2 overnight. On the next morning, thoroughly clean with ddH2O and leave all day in ddH2O. Finally, rinse a bit more to make sure any H2O2 residues are gone before using them again.
- Alternatively, replace for a new one.
[Proper positioning of the inflow and outflow in the recording chamber]
It is critical to find the best position for the outflow to ensure the bath level remains stable throughout your experiment.
- If using a needle as an outflow/suction, make sure the end is not completely immersed but it rather has half the tip immersed and half of it aspirating air. You may want to play around with bending, cutting, and pressing the tip to obtain the diameter and angle that best works in your set up.
- Then play around with the suction power (if using a pump that allows to independently change that) until you find the right ratio. You want to find the sweet spot where your bath level minimally oscillates and there are air bubbles being constantly formed in the outflow/suction. If the bath level oscillates, you may end up with problems in the baseline of your recordings.
- Having the inflow closer to the bottom of the recording chamber (i.e. lower) and the outflow further up (i.e. higher) can also help to keep the bath level stable.
- Another thing you can try is to have inflow and outflow exactly opposite of each other, so that the whole chamber is perfused equally and the liquid does not stop flowing in any part of it. This will make sure the whole slice is equally perfused with ACSF and any drugs you may use.
- You may also add two needles in between the tubing and ground it, to get rid of any pump-related noise.
- Finally, make sure the tube that aspirates the recording ACSF is not too close to the bubblestone, otherwise you will get bubbles getting into your bath.

Other considerations:
- Check the flow rate and reduce it to around 2-4 ml per minute during experiment. It is good practice to actually measure the flow by collecting liquid from the outflow for a minute and comparing the volume you get with the flow you have set in the system.
- Measure the time it takes for the solution to actually reach the recording chamber - this will be useful to know when bath-applying drugs in your experiments.
- Before placing a slice in the recording chamber, immerse your harp in the ACSF external solution and let it rest there for a bit with the pump on. Otherwise, if you try to place it completely dry it may repel the recording ACSF (and some times even the slice you are trying to hold in place).
- When you place a new brain slice into the bath make sure there are no air/gas bubbles in the tissue, or it may float away instead of sinking to the bottom of the recording chamber. Wait for the tissue to settle down gently and do not play with the section too much - gently position it with forceps or a brush by gentle pushing from sides, without pinching/pressing the tissue (avoid any mechanical stress).
- Place the harp on the slice making sure that the closed U end is facing the incoming flow, not the open end. This will minimise any disturbances on your recording by the inflow. Make sure the strings are at the right distance and tension to hold the tissue without cutting through it.
[Replacing the coverslip in the recording chamber]
- Making a new recording chamber (i.e. replacing the coverslip at the bottom) requires an overnight step to let the sealant harden, so it is probably wise to always have a spare one ready to go in the unlikely (or unavoidable) event of breaking the coverslip at the bottom of the recording chamber by crashing the objective on it.
- If, or when, that happens, make sure to stop the pump as soon as possible to minimise any spillage of ACSF on the condenser or any other optic elements. If you manage to stop it in time and have a replacement chamber, you can simply replace the broken one with the spare one, reposition the ground/inflow/outflow/thermistor, place a new slice, and continue with your experiments. Otherwise you may have to remove the condenser to clean it right away and dry it (if the ACSF dries there will be salts everywhere and will be harder to clean), place it back and re-do the Kohler.
- Once you are done for the day and are ready to fix the broken chamber, start by removing any sealant and coverslip with a blade and thoroughly clean the chamber with 70% ethanol and ddH2O.
- Once it is clean and dry, use a cotton bud to apply some sealant on the edges (e.g. Dow Corning 732 multi-purpose sealant, image below). Carefully mount a new coverslip and gently apply pressure to seal the gap between the chamber and the coverslip.
- With a clean cotton bud, clean the excess sealant without staining the coverslip (the light still needs to go through it to reach the brain slice) and leave the sealant harden overnight.
- The next morning, check that there is no leak by adding some water. Use ddH20 and a kimwipe to clean the coverslip and store the recording chamber as a spare.

[Chloriding the recording and ground electrodes]
In our system, a silver wire (0.35 mm diameter, Cat. No. AG005145, GoodFellow) coated with silver chloride (AgCl) is present inside the pipette and in contact with the intracellular solution, and either an Ag-AgCl pellet electrode or a coiled AgCl coated silver wire is used as bath electrode. The extracellular and intracellular solutions contain chloride ions to allow a reversible exchange of ions: Ag + Cl- <-> AgCl + e-. To ensure that this ionic exchange occurs at all times, the silver wires need to be regularly chlorided.
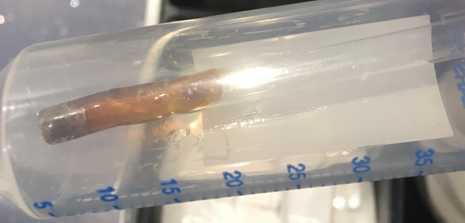
- Chloriding with solid silver chloride powder : first of all make sure you wear some gloves to clean the silver wire (you can carefully scrape off any dirt or residues with a blade), then scoop a bit of silver chloride powder (Cat. No. 204382-5G, Sigma Aldrich) with a glass pipette (the same type you use to make the pipette to pick up and transfer slices from one place to another, image below) and hold over the flame of a bunsen burner until it melts (the powder will become liquid and turn into a dark brown colour). Once it is melted, slide the silver wire quickly in and out so it gets coated with the liquid silver chloride. Once it solidifies again, you have a solid coating that lasts for much longer than with the other methods below.
- In order to avoid having to constantly re-chloride the ground (which will be necessary if using a coiled silver wire as a ground), a Ag-AgCl pellet and wire electrodes can be used (Cat. No. E206, Warner Instruments) as they are more stable than the coiled silver wire and there is no need to rechloride them every now and then. Once the ground or baseline turns unstable, all you have to do is switch the pellet for a new one (ideally have a spare one already soldered to a ground cable and pin connector compatible with the headstage ready to go).
- If you don't have ground pellets, you can make one with silver wire, but you will have to bleach/chloride it every now and then.Take a silver wire, fold it in half, and then folding it again (so that you are left with a kind of tetrode). You then roll it on its own and flame the tip until it melts and makes a tiny ball. You can then chloride/bleach it and solder it to a ground cable and it should work fine.
Alternative ways of chloriding the electrodes are:
- Chloriding with bleach : pour some bleach on an eppendorf tube that has a hole in the cap, insert the electrode, and leave overnight. Once properly chlorided it should change to a darker colour (the Cl- has then correctly deposited on the surface of the silver wire).
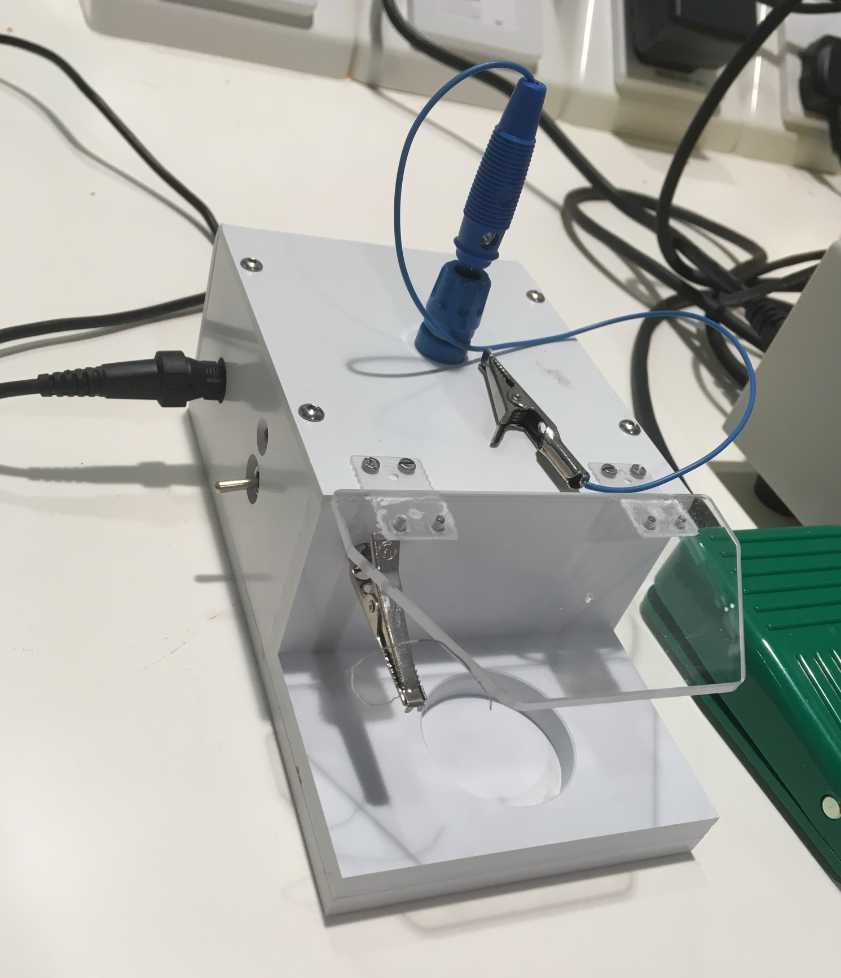
- Chloriding with 3M KCL solution and a battery : prepare a 3M KCl solution in a bijou vial, place on the machine (image below), connect the electrode you want to chloride to the crocodile clip above and a piece of cut silver wire to the other, place both electrodes in the 3M KCl solution making sure they are making contact with the crocodile clips but not with each other. Pull the switch (the light will go red) and let it run. It will switch off automatically once it is done after a few minutes. Again, if properly chlorided the electrode should have changed to a darker colour.
[Pipette holder]
- It is good practice to periodically take apart the holder to clean all the parts and replace the O-rings if necessary.
- Use gloves when taking it apart and assembling it back, to avoid depositing any grease from your skin.
- Clean all the parts with ddH2O and let dry overnight. Once dry, use an air duster to clean and remove any dust or water left.
- Put it back together [for the HEKA, see link & link].
[Setting up and adjusting a micromanipulator]
- Angle the micromanipulator to around 15-20 degrees, center it across all axis (i.e. if the axis can go to 25000, set it to 12500), and then mount a pipette and position the manipulator so the tip stands in the center of your bath chamber. This should give you maximal mobility.
- The tubing for applying pressure and suction should be as short as possible, and in general hard tubing would be better. A more elastic tube will give way and expand with the pressure, so you will more likely loose a bit of pressure and also your suction pulses will reach the cell a bit slower.
[Harp]
- The harp is usually a U-shaped piece of platinum wire with a few nylon strings evenly spaced (reasonably frequent and not too close or far apart) that hold the brain slice in place so that you can achieve recordings without the tissue being moved by the constant flow of ACSF or the pipette. Use 1 mm diameter platinum wire (0.1 m length, Cat. No. PT005155, GoodFellow) previously flattened and cut and shaped to the desired dimensions.
[Backfilling glass electrodes]
- Use a 2-20 μL pipette and microloader tips to fill microcapillaries with internal solution.
- Use enough solution to cover the tip of the silver wire that acts as recording electrode in the headstage, but don't overfill as this will increase the capacitance of your pipette.
- You may also use a lighter and a 200 μL tip to manually pull your own thin tip to backfill the glass electrodes.
[Liquid Junction potential]
Whenever you have different solutions in the recording pipette and the bath, there will be a charge separation across the junction between the two solutions, owing to the different ionic composition of each solution. You can either calculate the expected liquid junction potential for your two solutions or measure it.
Useful references:

