Preparation of Single Cell Suspensions of the Intra-Epithelial Layer and Lamina Propria from Human Intestinal Tissue
Steven B. Wells, Peter A. Szabo, Pranay Dogra, Joshua I. Gray, Daniel P. Caron, Yoonseung Lee, Rory Morrison-Colvin
Gut
Intestine
Jejunum
Ileum
Colon
GI
CD45
Lymphocytes
Myeloid
Isolation
Density Gradient
Ficoll
Immune
10x
scRNAseq
Flow cytometry
Leukocyte
Single cell suspension
T cell
Epithelium
Lamina propria
Abstract
This protocol describes a method for the isolation of the immune cells, structural and epithelial cells, and progenitors from the epithelial layer and the lamina propria of human gut sections of about one gram of tissue. By providing defined media formulations, volumes at each step, and a defined dilution factor for density centrifugation, it yields consistent single-cell suspensions across samples. This protocol can be used for any section of the intestinal tract from duodenum to distal colon.
Attachments
Steps
Preparing Mediums and Buffers
Create the following IMDM-FBS-PSQ Media in a 500mL bottle of IMDM by using the table below:
| A | B | C | D |
|---|---|---|---|
| Component | Volume (mL) | Starting Conc. | Final Conc. |
| IMDM | 500 | - | - |
| Penicillin-Streptomycin-Glutamine | 5 | 100X | 1X |
| FBS | 50 | 100% | 10% |
Table 1.
Create the following DPBS-FBS Solution in a bottle of DPBS by using the table below:
| A | B | C | D |
|---|---|---|---|
| Component | Volume (mL) | Starting Conc. | Final Conc. |
| DPBS | 500 | - | - |
| FBS | 25 | 100% | 5% |
Table 2.
Create the following IMDM-FBS-PSQ-EDTA-DTT Media in a 500mL bottle of IMDM by using the table below:
| A | B | C | D |
|---|---|---|---|
| Component | Volume (mL) | Starting Conc. | Final Conc. |
| IMDM | 500 | - | - |
| FBS | 50 | 100% | 10% |
| Penicillin-Streptomycin-Glutamine | 5 | 100X | 100X |
| EDTA | 10 | 0.5M | 10mM |
| DTT | 1 | 1M | 2mM |
Table 2.
Create the following DPBS-FBS-EDTA Solution in a bottle of DPBS by using the table below:
| A | B | C | D |
|---|---|---|---|
| Component | Volume (mL) | Starting Conc. | Final Conc. |
| DPBS | 500 | - | - |
| FBS | 25 | 100% | 5% |
| EDTA | 1 | 0.5M | 10mM |
Table 2.
Tissue Preparation
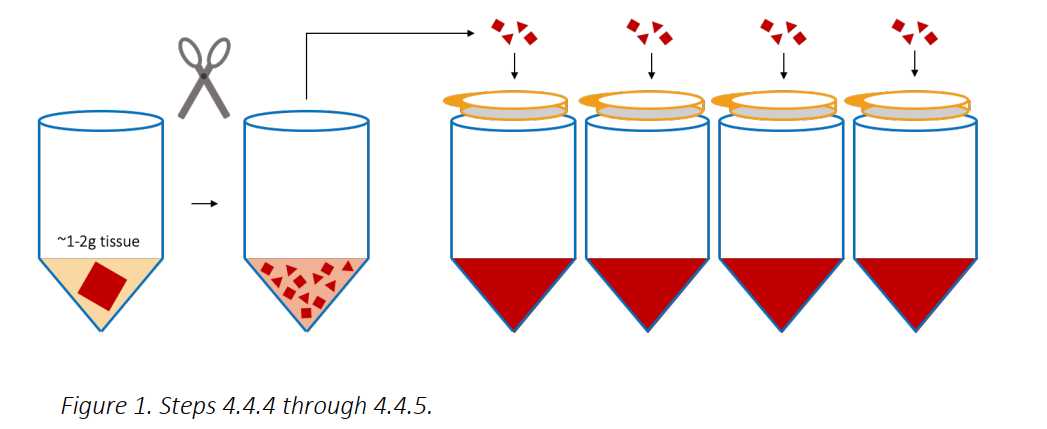
Use a surgical scissors to remove about 7cm-8cm of intestinal tissue section from the mysentary. Remove any remaining mysentary from the intestinal tissue.
Gently massage the chyme or fecal matter out of the tissue over a bucket.
Cut open the tissue on a tray containing cold DPBS-FBS Solution and add the tissue to a 250mL conical with 100mL of cold DPBS-FBS Solution and using a forceps gently agitate the tissue to remove yellow/brown chyme, fecal matter and/or mucus.
Discard the DPBS-FBS solution into a bucket, replace with 100mL of cold DPBS-FBS Solution and continue to wash the tissue until the DPBS-FBS Solution is no longer brown – when it has been successfully cleaned the DPBS-FBS Solution it should appear cloudy yellow/white. Depending upon how clean the tissue is this may take numerous washes (anywhere between 3-10, perhaps more).
Tissue Dissociation – Epithelial Stripping (IE Fraction)
Add 1±0.2 grams of the cleaned intestinal tissue to a 50mL centrifuge tube and record the weight below:
Total weight. __________g.
Add 20mL of Room temperature IMDM-FBS-PSQ-EDTA-DTT Media to the tissue-containing 50mL tube. Incubate on a shaker for 0h 30m 0s at 37°C.
Filter the cell suspension through a 100micromolar (µM) filter into a 50mL conical, rinse the tissue and the filter with 20mL of DPBS-FBS Solution. Set the cell suspension aside at 4°C. Place the remaining tissue into a back into its original 50mL conical.
Re-add 20mL IMDM-FBS-PSQ-EDTA-DTT Media to the tissue and incubate on a shaker for 0h 30m 0s at 37°C.
Filter the cell suspension through a 100micromolar (µM) filter into a 50mL conical, rinse the tissue and the filter with 20mL of DPBS-FBS Solution. Set the cell suspension aside at 4°C. Place the remaining tissue into a back into its original 50mL conical.
Tissue Dissociation – Lamina Propria Digestion (LP Fraction)
Add 5mL of Room temperature IMDM (NO ADDITIVES! Just the base media formulation) to the tube and use a scissors to chop the tissue into a fine “mash”.
Add 40mL of 37Room temperature IMDM (NO ADDITIVES) and spike in 0.400mL of Collagenase D, and 0.400mL of DNAse to the tube to begin the enzymatic digestion. Place on a shaker for 0h 30m 0s at 37°C.
Distribute and filter the mash of tissue over 100micromolar (µM) cell strainers above 50mL tubes (about 4 filters/gram of tissue).
Apply pressure with the black rubber bottom or the plastic end of a 10mL syringe plunger to any remaining, partially digested tissue on the cell strainers, and intermittently wash through with DPBS-FBS-EDTA Solution from a transfer pipet. When finished, combine the tubes of cell suspension and proceed to the next section.
Ficoll-Paque
Centrifuge the cell suspensions (EL and LP fractions) for 0h 10m 0s at 400x g,0h 0m 0s at 20°C.
Remove the EL and LP supernatants and combine the cell pellets down to a single 50mL, 1g/tube, keep the fractions distinct, add 10mL with 20Room temperature IMDM (NO ADDITIVES) .
Add 10µL of benzonase/1 gram of tissue to the EL and LP fractions and incubate at 37°C for 0h 30m 0s.
Add 15mL of IMDM (NO ADDITIVES) to the cell suspension, spike in 0.250mL of EDTA 0.5Molarity (M) 8.0 to all tubes.
Filter both the EL and LP cell suspensions through a 100micromolar (µM) cell strainer.
Layer 25mL of cell suspension (both IE and LP fractions) on top of 15mL of Ficoll-Paque Media PLUS.
Spin for 0h 20m 0s, 1200x g,0h 0m 0s at 20°C with 4 acceleration and 0 brake, evenly distribute the tubes across the entire rotor to prevent wobbling (use all four buckets if possible as opposed to just two).
For both fractions, remove the mononuclear cell layer with a transfer pipet and transfer to a separate 50mL tubes. Add cold DPBS-FBS-EDTA Solution to a final volume of 50mL and centrifuge the cell suspensions for 0h 10m 0s at 400x g,0h 0m 0s, 4°C.
Remove the supernatant and re-suspend the cell pellet in 50mL cold DPBS-FBS-EDTA Solution and centrifuge the cell suspension for 0h 10m 0s at 120x g,0h 0m 0s, 4°C.
Remove the supernatant and re-suspend the cell pellet in cold 10mL IMDM-FBS-PSQ Media.
Cell Count
IE Fraction - Count cells, and viability by using the NC-3000 cell counter. Calculate total viable cells and record below:
cell number: ________cells/mL, ________% viable
final volume: ________mL
𝑐𝑒𝑙𝑙 𝑛𝑢𝑚𝑏𝑒𝑟 (𝑐𝑒𝑙𝑙𝑠/𝑚𝐿) ∗ 𝑣𝑖𝑎𝑏𝑖𝑙𝑖𝑡𝑦(%) ∗ 𝑓𝑖𝑛𝑎𝑙 𝑣𝑜𝑙𝑢𝑚𝑒 (𝑚𝐿) = 𝑡𝑜𝑡𝑎𝑙 𝑣𝑖𝑎𝑏𝑙𝑒 𝑐𝑒𝑙𝑙𝑠
Total Viable Cells: _______
LP Fraction - Count cells, and viability by using the NC-3000 cell counter. Calculate total viable cells and record below:
cell number: ________cells/mL, ________% viable
final volume: ________mL
𝑐𝑒𝑙𝑙 𝑛𝑢𝑚𝑏𝑒𝑟 (𝑐𝑒𝑙𝑙𝑠/𝑚𝐿) ∗ 𝑣𝑖𝑎𝑏𝑖𝑙𝑖𝑡𝑦(%) ∗ 𝑓𝑖𝑛𝑎𝑙 𝑣𝑜𝑙𝑢𝑚𝑒(𝑚𝐿) = 𝑡𝑜𝑡𝑎𝑙 𝑣𝑖𝑎𝑏𝑙𝑒 𝑐𝑒𝑙𝑙𝑠
Total Viable Cells: ________
Freeze-down
( Optional QC ) Aliquot 2 x 106 cells to a 5mL Falcon tube and place on ice for subsequent flow cytometric analysis.
Aliquot cells for analysis or experimentation, and then freeze down cells in up to 5 x 106 aliquots using Cryostor CS10 Medium, a Mr. Frosty, and a-80°C freezer (1mL-1.5mL aliquots, round down to the nearest 5 million cells and discard/freeze/use any left over cells). Record the number of vials frozen: __________.