Preparation of Single Cell Suspension from Human Lymph Node Tissue
Steven B. Wells, Peter A. Szabo, Nora Lam, Maya M.L. Poon
Lymph node
CD45
Lymphocytes
Myeloid
Isolation
Density gradient
Ficoll
Immune
10x
scRNAseq
Flow cytometry
Leukocyte
Single cell suspension
T cell
Abstract
This protocol describes a method for the isolation of pan-lymphocytes, pan-myeloid cells, and progenitors from human lymph node tissue. By providing defined media formulations, volumes at each step, and a defined dilution factor for density centrifugation, it yields consistent single-cell suspensions across samples.
Attachments
Steps
Preparing Mediums and Buffers
Create the following IMDM-FBS-PSQ Media in a 500mL bottle of IMDM by using the table below:
| A | B | C | D |
|---|---|---|---|
| Component | Volume (mL) | Starting Conc. | Final Conc.* |
| IMDM | 500 | - | - |
| Penicillin-Streptomycin-Glutamine | 5 | 100X | 1X |
| FBS | 50 | 100% | 10% |
Table 1.*Final Concentration is approximate.
Create the following DPBS-FBS-EDTA Solution in a bottle of DPBS without calcium and magnesium by using the table below:
| A | B | C | D |
|---|---|---|---|
| Component | Volume (mL) | Starting Conc. | Final Conc.* |
| DPBS | 500 | - | - |
| FBS | 25 | 100% | 5% |
| EDTA | 1 | 0.5M | 1mM |
Table 2.*Final Concentration is approximate.
Tissue Dissociation
Clean lymph nodes of fat and connective tissue post dissection, record the site below.
Add up to 2 ± 10% grams of cleaned lymph node tissue to per 50mL centrifuge tube – record the total weight below.
__________g
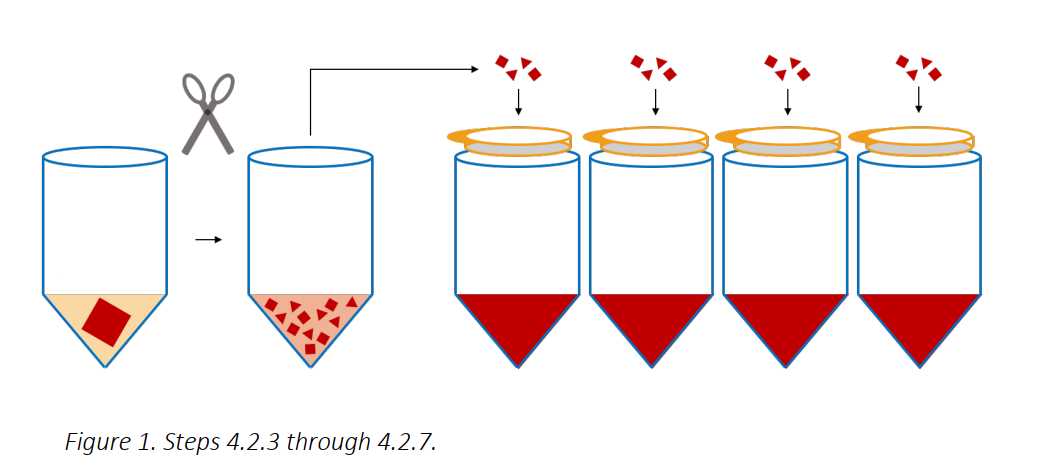
Add 5mL of room temperature IMDM (NO ADDITIVES! Just the base media formulation) to each tube and use a scissors to chop the tissue into a fine “mash”.
Add 35mL of room temperature IMDM (NO ADDITIVES! Just the base media formulation) and spike in 0.400mL of Collagenase D, and 0.400mL of DNAse to the tube to begin the enzymatic digestion. Place on a shaker or rotator for 0h 30m 0s at 37°C.
Distribute and filter the mash of tissue over 100micromolar (µM) cell strainers above 50mL tubes (about 4 filters/2 grams of tissue).
Apply pressure with the black rubber bottom or the plastic end of a 10mL syringe plunger to any remaining, partially digested tissue on the cell strainers, and intermittently wash through with DPBS-FBS-EDTA Solution from a transfer pipet – the aim is to push and wash through the tissue until only light pink/white/grey connective tissue remains. When finished, combine the tubes of cell suspension and proceed to the next section.
Ficoll-Paque
Centrifuge the cell suspensions for 0h 10m 0s at 400x g,0h 0m 0s at 20°C.
Remove the supernatants and combine the cell pellets down to a single 50mL tube, top to 50mL with 20Room temperature IMDM-FBS-PSQ Media, spike in 0.500mL of EDTA 0.5Molarity (M) 8.0.
Filter the cell suspension through a 100micromolar (µM) cell strainer.
In two 50mL tubes, layer 25mL of cell suspension on top of 15mL of Ficoll-Paque Media PLUS.
Spin for 0h 20m 0s, 1200x g,0h 0m 0s at 20°C with 4 acceleration and 0 brake, evenly distribute the tubes across the entire rotor to prevent wobbling (use all four buckets if possible as opposed to just two).
Remove the mononuclear cell layer from both tubes with a transfer pipet and combine in one 50mL tube. Add cold DPBS-FBS-EDTA Solution to a final volume of 50mL and centrifuge the cell suspension for 0h 10m 0s at 400x g,0h 0m 0s, 4°C.
Remove the supernatant and re-suspend the cell pellet in 50mL cold DPBS-FBS-EDTA Solution and centrifuge the cell suspension for 0h 10m 0s at 120x g,0h 0m 0s, 4°C.
Remove the supernatant and re-suspend the cell pellet in cold 10mL IMDM-FBS-PSQ Media.
Cell Count
Count cells, and viability by using the NC-3000 cell counter. Calculate total viable cells and record below:
cell number: ________cells/mL, ________% viable
final volume: ________mL
𝑐𝑒𝑙𝑙 𝑛𝑢𝑚𝑏𝑒𝑟 (𝑐𝑒𝑙𝑙𝑠/𝑚𝐿) ∗ 𝑣𝑖𝑎𝑏𝑖𝑙𝑖𝑡𝑦(%) ∗ 𝑓𝑖𝑛𝑎𝑙 𝑣𝑜𝑙𝑢𝑚𝑒(𝑚𝐿) = 𝑡𝑜𝑡𝑎𝑙 𝑣𝑖𝑎𝑏𝑙𝑒 𝑐𝑒𝑙𝑙𝑠
Total Viable Cells: ________
Analysis and Freeze-down
( Optional QC ) Aliquot 2 x 106 cells to a 5mL Falcon tube and place on ice for subsequent flow cytometric analysis.
Aliquot cells for analysis or experimentation, and then freeze down cells in up to 3 x 107 aliquots using Cryostor CS10 Medium, a Mr. Frosty, and a -80°C freezer (1mL-1.5mL aliquots, round down to the nearest 20 million cells and discard/freeze/use any left over cells). Record the number of vials frozen: __________.