Opentrons pipeline: gDNA bead cleanup
Jhakelin Reyes Vasquez, P. Sánchez-Vendizú, Gideon Erkenswick, Mrinalini Watsa
Abstract
This protocol is an automated pipeline to clean a plate of extracted DNA using SPRI bead cleanup. It is functional for both. It is typically used to clean up a portion of extracted DNA which has suspected impurities capable of affecting downstream PCR or sequencing protocols. This is often a side effect of extraction by magbeads with no spin-column based purifications. We have found this protocol to be effective on DNA extracted from blood, biopsy and feces in a wide range of Neotropical vertebrates.
This protocol was developed and optimised for the following:
- Platform: Opentrons OT-2 automated pipetting robot
- Kit: Ampure beads and home-brewed bead solutions
- Tips Used: 5 boxes (2 x 200uL Opentrons Filtered Tip boxes and 3 x 20uL Opentrons Filtered Tip boxes)
- Number of samples: 96
Before start
Clean the OT2 deck and walls with:
Steps
BEFORE STARTING
Materials:
Autoclave and UV the items you will use to ensure sterility. Some items can be autoclaved and reused as indicated below.
| A | B | C |
|---|---|---|
| Item | # | Status |
| Opentrons 200µL Filter Tips | 2 | NEW |
| Opentrons 20µL Filter Tips | 3 | NEW |
| NEST 1-Well Reservoirs, 195 mL | 1 | REUSED |
| NEST 12-Well Reservoirs, 15 mL | 1 | REUSED |
| Nest skirted PCR Plate | 1 | NEW |
| 96-Well PCR Plate Non-skirt, 200µl | 1 | NEW |
| Axygen™ PCR Tube Storage Racks | 1 | NEW |
| Polyester plate seal | 1 | NEW |
| 2ml microcentrifugue tubes | 2 | NEW |
Opentrons Equipment List
Equipment
| Value | Label |
|---|---|
| OT-2 | NAME |
| Liquid handler | TYPE |
| Opentrons | BRAND |
| OT-2 | SKU |
| https://opentrons.com/ | LINK |
On the right pipette mount use the P300M
Equipment
| Value | Label |
|---|---|
| OT-2 8 Channel Electronic Pipette | NAME |
| Pipette | TYPE |
| Opentrons | BRAND |
| P300M | SKU |
On the left pipette mount use the P20M
Equipment
| Value | Label |
|---|---|
| OT-2 Single Channel Electronic Pipette | NAME |
| Pipette | TYPE |
| Opentrons | BRAND |
| P20S | SKU |
Reagents:
Prepare all reagents in advance:
-
-
-
, freshly prepared -
We begin with a cleanup sample volume of 15uL. This allows you to cleanup a small volume, but larger volumes are easily cleaned with the same protocol. Simply add water 1:1, and then proceed.
| A | B | C | D |
|---|---|---|---|
| Ingredient | Amount per sample | Amount per 96 samples | Notes |
| Water | 15 | =15*96 | |
| SPRI beads | =1521.2 | =1521.2*96 | 1.2x water+sample |
| 70% ethanol | 250 | =250*96 |
Add water in a 1:1 ratio by volume of water to samples.
Add beads in a 1: 1.2 ratio (sample: beads) allowing for the fact that the sample contains both sample +water from step 2.1.
Each sample will be washed twice with freshly prepared Ethanol 70%.
Before loading your protocol, load this labware file into your Opentrons app: denville_96_axygenbase_200ul.json
This labware definition allows us to use a nonskirted plate in the Opentrons by inserting it into a skirted plate, and also allows us to use a 200uL plate (where our skirted plates that clip in are only 100uL. Feel free to replace with your own labware here ).
Load this python file to the Opentrons app: cleanup_gDNA_v4.3.py
Arrange the OT-2 deck
Number of samples: 96
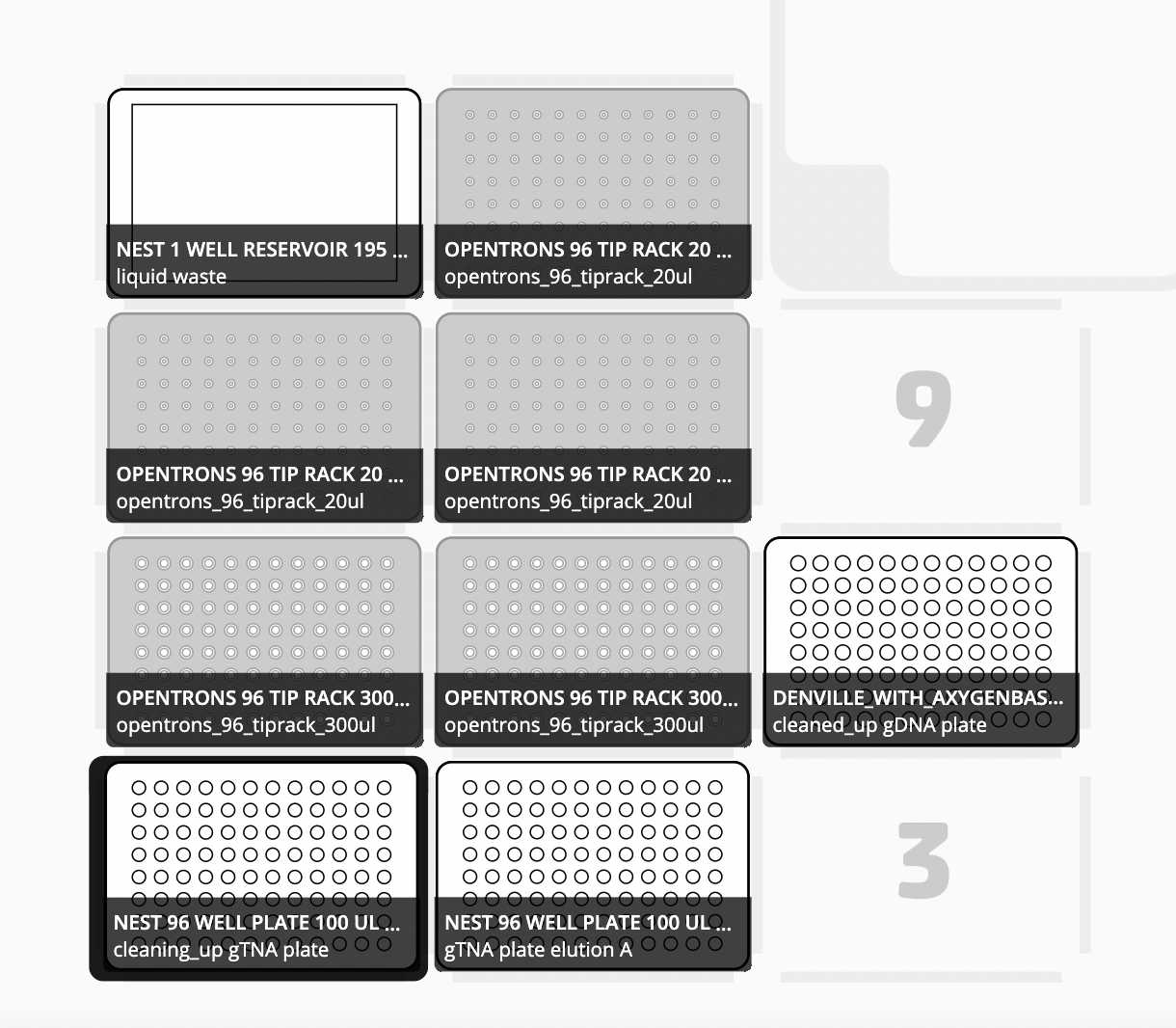
Slot 1: Opentrons Magnetic Module with Nest skirted PCR Plate empty (to receive cleaned DNA)
Slot 2: Nest skirted PCR Plate with TNA
Slot 3: NEST 12-Well Reservoirs, 15 mL with reagents preloaded in the following order:
| A | B | C | D | E | F | G | H | I | J | K | L | M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Channel # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Contents | SPRI BEADS | ALCOHOL | ALCOHOL | WATER |
Ingredients plan for a 12-well reservoir
Slot 4: Opentrons 200µL Filter Tips
Slot 5: Opentrons 200µL Filter Tips
Slot 6: Axygen™ PCR Tube Storage Rack with 96-Well PCR Plate Non-skirt, 200µl
Slot 8: Opentrons 200µL Filter Tips
Slot 9: Opentrons 200µL Filter Tips
Slot 10: NEST 1-Well Reservoirs, 195 mL (for waste)
Slot 11: Opentrons 200µL Filter Tips
Calibrate the deck. Follow the onscreen instructions.
OT2 SCRIPT DEFINITIONS
Definition of samples and labwares:
gTNA samples
Samples that will be cleaned and are in the elution plate A from previous TNA extraction.
Position: Slot 2 , Nest skirted PCR Plate with TNA
Name in the Deck : gTNA plate elution A
Labware name in the protocol: gTNA_plate_A
Sample name in the script : gTNA_samples
Samples to be cleaned
Samples that are in the magnet to be cleaned.
Position : Slot 1 , Nest skirted PCR Plate in Opentrons Magnetic Module
Name in the Deck : to_be_cleaned gTNA plate
Labware name in the script protocol : mag_plate
Sample name in the script : samples_to_be_cleaned
Cleaned samples
Samples that have been cleaned and will be eluted in the clean up plate.
Position: Slot 6 , Axygen™ PCR Tube Storage Rack with 96-Well PCR Plate Non-skirt, 200µl
Name in the Deck : cleaned_up gDNA plate
Labware name in the protocol: clean_up_plate
Sample name in the script : cleaned_samples
Protocol variables definition
This protocol is written to use 15µL of gTNA sample + 15µL of water to reach 30µL as final volume to start the clean up process for 96 samples. Therefore, if you want to modify the volumes and sample number just open the script in a text editor program, search and modify the values in the third line of the script:
"sample_number": 96 → Indicates the number of samples that you will process.
"gTNA_volume": 15 → Volume of gDNA that will be cleaned.
"bead_ratio": 1.2 → Ratio of beads volume
"elution_buffer_volume": 15 → Volume of water for elution
"incubation_time": 6 → Time in minutes for incubation of beads with the sample
"pelleting_time": 6 → Time with magnet engaged.
"drying_time": 5 → Time to the let alcohol evaporate
OT2 Clean up Protocol
Protocol
Transferring water to the gTNA plate
15µL of
The first column of tips in Slot 11 is used for dispensing water to all the columns.
Removing the 2nd ethanol wash
After an incubation of 0h 0m 30s, the supernatant is removed in two steps very gently to avoid removing settled beads. Supernatant is discarded in the Liquid waste NEST 1-Well Reservoirs, 195 mL in Slot 10.
Tips in Slot 4 are used for this step. Each column of tips is used for one column of samples.
Removing any remaining ethanol
30µL of remaining ethanol is removed very gently to avoid removing settled beads. Supernatant is discarded in Liquid waste NEST 1-Well Reservoirs, 195 mL in Slot 10.
Tips in Slot 4 are used for this step. Each column of tips is used for one column of samples.
Drying the beads
A pause of 0h 5m 0s is set to allow beads to dry at Room temperature to evaporate remaining ethanol.
Adding elution buffer or water
Disengaged the Opentrons Magnetic Module.
15µL of
Tips in the Slot 7 are used for this step. Each column of tips is used for each column of samples.
Mixing the beads
This is the second mixing step of water and sample before elution. The first column will be mixed, then the second and so on to the 12th column
The whole process is approximately 0h 10m 0s long.
Tips in the Slot 7 are used for this step.
Binding beads to the magnet
The Opentrons Magnetic Module is engaged for 0h 6m 0s to allow beads to pellet.
Elution of final DNA
15µL of cleaned gTNA are transferred from the to_be_cleaned gTNA plate in Slot 1 to the
cleaned_up gTNA plate in Slot 6.
Tips in the Slot 8 are used for this step. Each column of tips is used for each column of samples.
Storage of cleaned gDNA
Cover the cleaned_up gTNA plate with a plate seal and store at 4°C for use or -20°C for long term storage.
Transferring gTNA to the gTNA plate
15µL of
Tips in Slot 11 are used for this step.
Dispensing SPRI beads
36µL of SPRI beads are dispensed from Well 1 in the NEST 12-Well Reservoirs, 15 mL in Slot 3 to the to_be_cleaned gTNA plate in Slot 1.
The first column of tips in Slot 5 is used for dispensing SPRI beads to all the columns.
Mixing samples and beads
Two mixing steps are defined in the script. The first column will be mixed, then the second and so on to the 12th column, then it will be repeated. The whole process is approximately 0h 15m 0s long.
Tips in Slot 4 are used for this step. Each column of tips is used to mix each column of samples.
Allowing beads to settle on the magnet
The Opentrons Magnetic Module is engaged for 0h 6m 0s to allow beads settle.
Removing the supernatant
The supernatant is removed in two steps very gently to avoid removing settled beads. Supernatant is discarded in the Liquid waste NEST 1-Well Reservoir, 195 mL in Slot 10.
Tips in Slot 4 are used for this step. Each column of tips is used for one column of samples.
The first washing step
100µL of
The second column of tips in Slot 5 is used for dispensing SPRI beads to all the columns.
Removing the 1st ethanol wash
After an incubation of 0h 0m 30s, the supernatant is removed in two steps very gently to avoid removing settled beads. Supernatant is discarded in the liquid waste NEST 1-Well Reservoirs, 195 mL in Slot 10.
Tips in Slot 4 are used for this step. Each column of tips is used for one column of samples.
The second washing step
100µL of
The second column of tips in Slot 5 is used for dispensing SPRI beads to all the columns.
After finishing the protocol
Clean the OT2 deck and walls with:
Clean OT2 module with:
Air dry OT2 robot and modules.

