Opentrons Pipeline: DNA Extraction with the Omega Biotek Stool Kit
Kristina N Vsevolodova, Gideon Erkenswick, Mrinalini Watsa
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
This protocol is an automated pipeline to extract a full 96-well plate of DNA from stool lysates prepared in two separate plates. Lysing of the stool can be performed as you wish, depending on species and their diet. The protocol itself begins after lysates have been created.
This protocol was developed and optimized for the following:
Platform: Opentrons OT-2 automated pipetting robot
Kit: Omega Biotek
Tips Used: 4 boxes (4 x 200uL Opentrons Filtered Tip boxes)
Recommended number of samples: 96
Before start
Clean the OT2 deck and walls with:
Steps
Before starting
Ingredients
OR separate reagents (not including lysis reagents):
For the 1x96 Kit
Dilute SPM Buffer with 70mL Room temperature.
Dilute VHB Buffer with 28mL Room temperature.
For the 4x96 Kit
Dilute SPM Buffer with 70mL Room temperature.
Dilute VHB Buffer with 112mL Room temperature.
Materials
5x Nest 50mL Falcon Tubes for holding accurate reagent amounts before starting protocol.
4x Opentrons 200µL Filter Tips
1x VWR 96 Deep Well Plates 1mL for 300µL of lysate.
2x NEST 1-Well Reservoirs, 195 mL for extraction waste collection during protocol.
2x NEST 12-Well Reservoirs, 15 mL for holding reagents during protocol.
1x 96-Well PCR Plate Non-skirt, 200µl for 200µL of lysate.
3x Nest skirted PCR Plate for holding non-skirt plate and for final elutions.
2x Aluminium Seals
6x 2mL Tubes
100-1000µL pipette
1000µL pipette tips
Incubator or water bath that can reach 70°C or more
Autoclave the NEST 1-Well Reservoirs, 195 mL and NEST 12-Well Reservoirs, 15 mL before use. These can be rinsed and autoclaved and reused and need not be purchased new for each extraction. Slight yellowing of product can occur, but we do not see that it affects final outcomes in any discernible way.
Opentrons Equipment List
Equipment
| Value | Label |
|---|---|
| OT-2 | NAME |
| Liquid handler | TYPE |
| Opentrons | BRAND |
| OT-2 | SKU |
| https://opentrons.com/ | LINK |
On the right pipette mount use the P300M
Equipment
| Value | Label |
|---|---|
| OT-2 8 Channel Electronic Pipette | NAME |
| Pipette | TYPE |
| Opentrons | BRAND |
| P300M | SKU |
Magnetic Module to place in Slot 7
Equipment
| Value | Label |
|---|---|
| OT-2 Magnetic Module GEN2 | NAME |
| Module | TYPE |
| Opentrons | BRAND |
| 999-00098 | SKU |
Prepare reagents
After the reagents are properly diluted and materials are ready, prepare the following amounts:
| A | B | C | D |
|---|---|---|---|
| Item Name | Amount per sample [uL] | Amount for 96 samples [uL] | Amount for 96 samples * 1.1 overage[uL] |
| Mag-bind Bead Particles CH Round 1 | 10 | =10*96 | 1056 |
| XP2 Binding Buffer Round 1 | 300 | =300*96 | 31680 |
| Mag-bind Bead Particles CH Round 2 | 10 | =10*96 | 1056 |
| XP2 Binding Buffer Round 2 | 300 | =300*96 | 31680 |
| Wash 1: VHB Buffer | 400 | 38400 | 42240 |
| Wash 2: SPM Buffer | 400 | =400*96 | 42240 |
| Wash 3: SPM Buffer | 400 | =400*96 | 42240 |
| Elution Buffer | 100 | 9600 | 10560 |
Fill one Nest 50mL Falcon Tube with the amount of Room temperature. Split between two tubes if needed.
Fill one Nest 50mL Falcon Tube with the amount of Room temperature. Split between two tubes if needed.
Fill one Nest 50mL Falcon Tube with the volume indicated in the table with wash 1: Room temperature.
Fill one Nest 50mL Falcon Tube with the volume indicated in the table with wash 2: Room temperature.
Fill one Nest 50mL Falcon Tube with the volume indicated in the table with wash 3: Room temperature.
Distribute
Set an incubator or water bath to 70°C and heat70°C.
OT-2 script definitions
Definition of samples and labware:
Lysed Sample Plate 2 200uL
Remainder of lysed samples that will be added to Slot 7 in second round of bead incubation.
Position: Slot 2, 96-Well PCR Plate Non-skirt, 200µl with 200uL of sample lysis on top of an empty Nest skirted PCR Plate (used as a base)
Name in the Deck: Lysis plate 2
Labware name in the protocol: denvillewithaxygenbase_96_wellplate_200ul
Sample name in the script: lysate
Lysed Sample Plate 1 300uL
Lysed samples that will undergo the first round of bead incubation.
Position: Slot 7, Opentrons Magnetic Module with VWR 96-Well Deep Well Plate full of 300uL of sample lysis on top
Name in the Deck: Lysis plate 1
Labware name in the protocol: vwr_96_wellplate_1000ul
Sample name in the script: magsamps
Sample Elution 1
Samples that have been eluted from the beads for the first round elution 1.
Position: Slot 3, Empty Nest skirted PCR Plate (to receive elution 1)
Name in the Deck: Sample Elution Plate 1
Labware name in the protocol: nest_96_wellplate_100ul_pcr_full_skirt
Sample name in the script: eluates
Sample Elution 2
Samples that have been eluted from the beads for the second round elution 2.
Position: Slot 2, 96-Well PCR Plate Non-skirt, 200µl with 200uL of sample lysis on top of an empty Nest skirted PCR Plate is replaced with new Nest skirted PCR Plate (to receive elution 2)
Name in the Deck: Sample Elution Plate 2
Labware name in the protocol: nest_96_wellplate_100ul_pcr_full_skirt
Sample name in the script: eluates2
Prepare the OT-2
Before loading your protocol, load the following labware files into your Opentrons app: denville_96_axygenbase_200ul.json
This labware definition allows us to use a non-skirted plate in the Opentrons app by inserting it into a skirted plate, and also allows us to use a 200uL plate (where our skirted plates that clip in are only 100uL. Feel free to replace with your own labware here).
This labware definition is for the 1mL deepwell plate from VWR. Note the rounded wells work well with the magnet.
Load this python file to the Opentrons app: OT2_Omegabiotekfecal_v4.0.py
Definition of Protocol Variables:
This protocol is written per column, best working for multiples of 8. Therefore, if you want to modify the sample number just open the script in a text editor program, and modify the following value in line 3 of the script:
"numSamps": 96 → Indicates the number of samples that you will process.
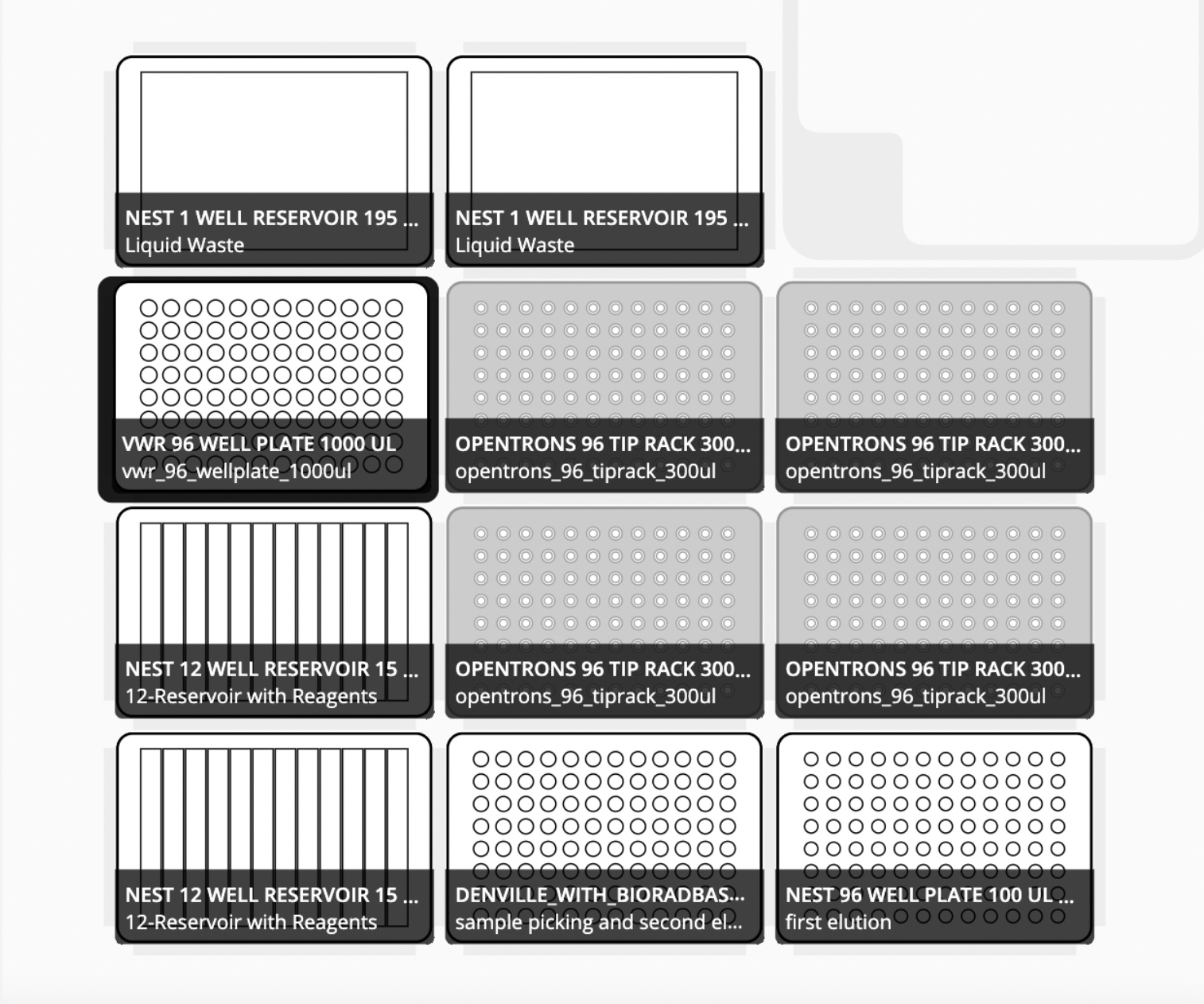
Arrange the OT-2 deck
Slot 1: NEST 12-Well Reservoirs, 15 mL with reagents preloaded in the following order:
| A | B | C | D | E | F | G | H | I | J | K | L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Well 1 | Well 2 | Well 3 | Well 4 | Well 5 | Well 6 | Well 7 | Well 8 | Well 9 | Well 10 | Well 11 | Well 12 |
| Wash 3: SPM Buffer | Wash 3: SPM Buffer | Wash 3: SPM Buffer | Wash 3: SPM Buffer | EMPTY | EMPTY | EMPTY | EMPTY | EMPTY | EMPTY | EMPTY | Elution Buffer (when prompted) |
Slot 2: 96-Well PCR Plate Non-skirt, 200µl with 200uL of sample lysis on top of an empty Nest skirted PCR Plate
Slot 3 : Empty Nest skirted PCR Plate (to receive elution 1)
Slot 4: NEST 12-Well Reservoirs, 15 mL with reagents preloaded in the following order:
| A | B | C | D | E | F | G | H | I | J | K | L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Well 1 | Well 2 | Well 3 | Well 4 | Well 5 | Well 6 | Well 7 | Well 8 | Well 9 | Well 10 | Well 11 | Well 12 |
| XP2 Binding Buffer and Magbind Particles Round 1 then 2 | XP2 Binding Buffer and Magbind Particles Round 1 then 2 | XP2 Binding Buffer and Magbind Particles Round 1 then 2 | EMPTY | Wash 1: VHB Buffer | Wash 1: VHB Buffer | Wash 1: VHB Buffer | Wash 1: VHB Buffer | Wash 2: SPM Buffer | Wash 2: SPM Buffer | Wash 2: SPM Buffer | Wash 2: SPM Buffer |
Slot 5: Opentrons 200µL Filter Tips
Slot 6: Opentrons 200µL Filter Tips
Slot 7: Opentrons Magnetic Module with VWR 96-Well Deep Well Plate full of 300uL of sample lysis on top
Slot 8: Opentrons 200µL Filter Tips
Slot 9: Opentrons 200µL Filter Tips
Slot 10: NEST 1-Well Reservoirs, 195 mL (for waste)
Slot 11: NEST 1-Well Reservoirs, 195 mL (for waste)
Run the OT-2 protocol
Adding SPM Buffer to Lysed Sample Plate 1 for wash 2
Column 3 of tips in Slot 5 will transfer 133µL transfer steps to each sample in Lysed Sample Plate 1 in Slot 7 without touching the lysates. The tips will then be dropped into the waste container.
Mixing SPM with Lysed Sample Plate 1
Column 2 of tips in Slot 8 will align with column 1 in Slot 7 to mix the sample by aspirating and dispensing 10µL. The tips will then be returned to their original starting point. Each subsequent column of tips will continue on the same pattern until all of the samples are mixed.
Incubating VHB Buffer and lysate
The Opentrons Magnetic Module is engaged and incubates the mixed samples for 0h 4m 0s.
Removing the supernatant from the wash
The supernatant is removed in two steps very gently to avoid removing settled beads. Supernatant is discarded in the Liquid waste NEST 1-Well Reservoir, 195 mL in Slot 10. Column 2 of tips in Slot 8 will align with column 1 in Slot 7 to remove the supernatant and then will return the tips back to their original starting point. Each subsequent column of tips will continue on the same pattern until all of the samples have their supernatant removed.
Adding SPM Buffer to Lysed Sample Plate 1 for wash 3
Column 4 of tips in Slot 5 will transfer 133µL transfer steps to each sample in Lysed Sample Plate 1 in Slot 7 without touching the lysates. The tips will then be dropped into the waste container.
Mixing SPM with Lysed Sample Plate 1
Column 2 of tips in Slot 8 will align with column 1 in Slot 7 to mix the sample by aspirating and dispensing 10µL. The tips will then be returned to their original starting point. Each subsequent column of tips will continue on the same pattern until all of the samples are mixed.
Incubating SPM Buffer and lysate
The Opentrons Magnetic Module is engaged and incubates the mixed samples for 0h 4m 0s.
Removing the supernatant from the wash
The supernatant is removed in two steps very gently to avoid removing settled beads. Supernatant is discarded in the Liquid waste NEST 1-Well Reservoir, 195 mL in Slot 11. Column 2 of tips in Slot 8 will align with column 1 in Slot 7 to remove the supernatant and then will return the tips back to their original starting point. Each subsequent column of tips will continue on the same pattern until all of the samples have their supernatant removed.
Allowing beads to air dry
The Opentrons Magnetic Module is engaged for 0h 1m 0s to allow the
Removing excess wash buffer
Column 2 of tips in Slot 8 will align with column 1 in Slot 7 to remove excess wash by aspirating 10µL and dispensing into the Liquid waste NEST 1-Well Reservoir, 195 mL in Slot 11. The tips will then be returned to their original starting point. Each subsequent column of tips will continue on the same pattern until all of the wash buffer is removed.
Calibrate the deck if needed. Follow the on screen instructions.
Allowing beads to air dry
The Opentrons Magnetic Module remains engaged for 0h 2m 0s to allow the
Adding elution buffer to Lysed Sample Plate 1 for Elution 1
The user must remove the 2.0mL tubes with 70°C incubator and pour 4 of these tubes into well 12 in Slot 1 .
Column 5 of tips in Slot 5 will transfer 60µL of 40µL. The tips will then be dispensed into the waste container. Each subsequent column of tips will continue on the same pattern until all of the samples are mixed with warmed elution buffer.
Incubating the beads with DNA in elution buffer
The protocol is paused for 0h 15m 0s to allow for the Room temperature.
Allowing beads to settle on the magnet
The Opentrons Magnetic Module is engaged for 0h 2m 0s to give the
Transferring each sample elution to Sample Elution Plate 1
Column 6 of tips in Slot 5 will transfer 60µL of each eluate to a new, clean Nest skirted PCR Plate in Slot 3 . Each subsequent column of tips will continue on the same pattern until all of the sample eluates are transferred, extending into the tips in Slot 9 . The tips will be returned to the tip boxes to be reused for Elution 2.
The Opentrons Magnetic Module is disengaged.
Adding elution buffer to Lysed Sample Plate 1 for Elution 2
The user must remove the 2.0mL tubes with 70°C incubator and pour the remaining 2 tubes into well 12 in Slot 1 . They must also replace the 96-Well PCR Plate Non-skirt, 200µl now empty of sample lysis on top of an empty Nest skirted PCR Plate in Slot 2 with a new, clean Nest skirted PCR Plate to receive Elution 2
Column 5 of tips in Slot 9 will transfer 40µL of 20µL. The tips will then be dispensed into the waste container. Each subsequent column of tips will continue on the same pattern until all of the samples are mixed with warmed buffer, extending into the tips in Slot 6 .
Incubating the beads with DNA in elution buffer
The protocol is paused for 0h 15m 0s to allow for theRoom temperature.
Allowing beads to settle on the magnet
The Opentrons Magnetic Module is engaged for 0h 2m 0s to give the
Transferring each sample elution to Sample Elution Plate 2
The same tips that were used for Elution 1 will transfer 40µL of each eluate to a new, clean Nest skirted PCR Plate in Slot 2 . Each subsequent column of tips will continue on the same pattern until all of the sample eluates are transferred. The tips will be dispensed into the waste container.
The Opentrons Magnetic Module is disengaged.
Storage of Sample Elution Plates 1 and 2
Cover the plates with an aluminium plate seal and store at 4°C for use or -20°C for long term storage.
Mixing Buffer and Particles with Lysed Sample Plate 1
Column 2 of tips in Slot 8 will align with column 1 in Slot 7 to mix the sample by aspirating and dispensing 10µL. The tips will then be returned to their original starting point. Each subsequent column of tips will continue on the same pattern until all of the samples are mixed.
QC of Sample Elution Plates 1 and 2
See QC Note in Step 1 for options.
Allowing beads to settle on the magnet
The Opentrons Magnetic Module is engaged and incubates the mixed samples for 0h 10m 0s.
Removing the supernatant
The supernatant is removed in two steps very gently to avoid removing settled beads. Supernatant is discarded in the Liquid waste NEST 1-Well Reservoir, 195 mL in Slot 10. Column 2 of tips in Slot 8 will align with column 1 in Slot 7 to remove the supernatant and then will return the tips back to their original starting point. Each subsequent column of tips will continue on the same pattern until all of the samples have their supernatant removed.
Adding VHB Buffer to Lysed Sample Plate 1 for wash 1
Column 2 of tips in Slot 5 will transfer 133µL transfer steps to each sample in Lysed Sample Plate 1 in Slot 7 without touching the lysates. The tips will then be dropped into the waste container.
Mixing VHB with Lysed Sample Plate 1
Column 2 of tips in Slot 8 will align with column 1 in Slot 7 to mix the sample by aspirating and dispensing 10µL. The tips will then be returned to their original starting point. Each subsequent column of tips will continue on the same pattern until all of the samples are mixed.
Incubating VHB Buffer and lysate
The Opentrons Magnetic Module is engaged and incubates the mixed samples for 0h 4m 0s.
Removing the supernatant from the wash
The supernatant is removed in two steps very gently to avoid removing settled beads. Supernatant is discarded in the Liquid waste NEST 1-Well Reservoir, 195 mL in Slot 10. Column 2 of tips in Slot 8 will align with column 1 in Slot 7 to remove the supernatant and then will return the tips back to their original starting point. Each subsequent column of tips will continue on the same pattern until all of the samples have their supernatant removed.
After finishing the protocol
Clean the OT2 deck and walls with:
Clean OT2 module with:
Air dry OT2 robot and modules.

