Nigrostriatal organotypic cultures to study neuromelanin accumulation in dopaminergic circuits
Mario Fernandez-Ballester, Federico N. Soria
Abstract
In this protocol we describe the preparation and maintenance of rat organotypic cultures from parasagittal brain slices. We use a 13º slicing angle and a customized cocktail of small molecules and growth factors, to maximize the integrity of the nigrostriatal pathway and ensure the survival of dopaminergic neurons, respectively. The slices maintain the basic cytoarchitecture of the brain, including glia and the extracellular matrix (not including vessels nor immune system). The cultures can be used to study dopaminergic degeneration, cell-cell and cell-matrix interactions and are particularly suitable for AAV-mediated overexpression of transgenes, time-lapse live imaging and longitudinal studies.
Steps
Culture preparation
Prepare a 6-well culture plate with Millipore 0.4 µm culture inserts (see Materials) placed on top of 1 mL of Pro-dopaminergic organotypic medium (see Materials) per well. Incubate it at 37°C and 5% CO2 to warm up.
Prepare a p100 petri dish with 25 mL of Hank’s Balanced Salt solution without Ca2+ and Mg2+(ThermoFisher) supplemented with D-Glucose 36 mM (Gibco) and a p60 petri dish with 5 mL of the same dissection solution,and keep them On ice .
Desinfect all surfaces and dissection tools. Prepare the McIllwain Tissue Chopper (see Materials) with a fresh blade and clean holder plates.
Euthanize P10-P12 rat pups by quick decapitation using large scissors. Separate the head skin with a scalpel and expose the skull. Cut the skull through the middle line with fine scissors. Use forceps to pull apart the skull and expose the brain.
Remove the brain with a spatula and transfer to a filter paper to remove moist. Place the brain carefully into a p35 petri dish with a warm 4% solution of LM-Agarose (see Materials). Temperature of the agarose solution should be no more than 37°C .
Let the agarose gellify On ice for a few minutes.
As soon as the agarose is stiff, crop with a blade a block of agarose containing the brain. Paste the block with cyanocrilate glue to the holder plate so the ventral part is in contact with the plate.
Position the plate in the chopper with the brain midline oriented 13º from the blade (see Image).
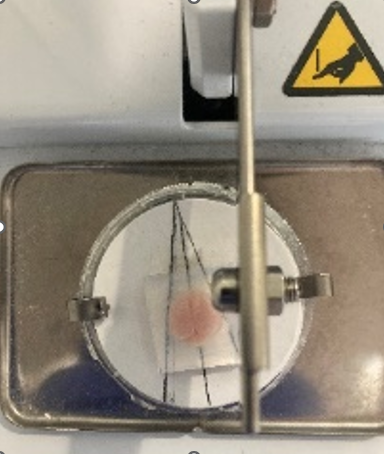
Obtain sagittal slices of 350 μm width at 13º angle. When the blade arrives to the midline, rotate the holder plate so the blade is aligned with the rightmost line and section again.
Separate the agarose block from the plate with a scalpel, avoiding damage to the ventral part. Pour the slices into the p100 petri dish with HBSS (from step 2).
Under the dissection microscope, remove the agarose and separate the slices with a couple of spatulas (preferentially plastic-ones, which can be custom crafted cutting the back of a Pasteur pipette). Only the 2nd or 3rd slice lateral from the midline contain the full nigrostriatal pathway. Transfer the selected slices to the p60 petri dish for to wash them (you can use an inverted glass pipette with a suction rubber bulb to minimize damaging of the slices).
Transfer the slice to the preheated culture inserts with medium using the inverted glass pipette with a suction rubber bulb. Each insert can contain only one nigrostriatal slice. Remove excess HBSS with a pipette and incubate the slices at 37°C and 5% CO2.
Maintenance
At DIV1 (24h after culture preparation), replace the feeding medium with 1 mL of the same medium but adding antimitotic 4.4 uM AraC (Sigma-Aldrich).
At DIV3, replace the AraC-containing medium with fresh medium and add 1 uL of AAV9-CMV-hTyr (1012 vg/mL) over the substantia nigra. Do not touch the tissue.
For culture maintenance, change medium 3-times per week (small molecules and growth factors have to be added fresh in each medium change). Slices can survive in culture more than 30 days.
Neuromelanin observation
At 10 DIV, neuromelanin spots will appear in the substantia nigra as brown spots visible with the naked eye.
At 15 DIV, the hTyr expression is maximal and neuromelanin accumulation can be seen all over the substantia nigra and connected regions (the nigrostriatal pathway becomes brown). Since the slices do not flatten and cell density is higher, any fluorescence imaging should be done in confocal or two-photon setups where optical sectioning can be achieved. Neuromelanin should be ideally imaged with a color camera, to ensure that the accumulate is brown (neuromelanin) and not black/gray (cell death).

