Modeling acute visceral pain in adult zebrafish
Denis Rosemberg, Fabiano Costa
zebrafish
nociception
acute abdominal pain
abnormal body curvature
acetic acid
pharmacological manipulations
opioid system
analgesic properties
Abstract
This protocol describes a reliable procedure for assessing acute pain responses in adult zebrafish ( Danio rerio ) based on the abdominal writhing-like phenotype following a single intraperitoneal injection of acetic acid. The method is an inexpensive, fast, and easy-to-use protocol for measuring pain-like responses in adult zebrafish. The protocol involves five steps: analyses of baseline behavior, drug injection, post-injection recordings of behavior, euthanasia, and data analyses/interpretation. Intraperitoneal injection of 2.5–5.0% acetic acid elicits a robust pain-like behavior by changing zebrafish body curvature, which can be easily quantified using freely available imaging software. This response is sensitive to pharmacological manipulations, as morphine prevents altered body curvature, while naloxone blocks these effects. Pretreatment with diclofenac sodium (a non-steroidal anti-inflammatory drug commonly used as an analgesic) also prevents writhing-like behavior. Demonstrating high predictive and face validity, this unbiased protocol can be performed over the course of ~2 days, enabling a reliable assessment of acute pain-like responses in zebrafish, thus fostering in-depth analyses of complex pain-related mechanisms and anti-pain drug discovery.
Before start
As subjects, use adult zebrafish (~50% male and female ratio, 4–6 months old). Heterogeneous wild-type ( e.g. , short-fin), specific ( e.g. , AB, TU) or genetically modified zebrafish strains can be used. While using the same-batch fish cohorts is desirable, fish from different batches still provide highly consistent data. For testing, animals must be randomly separated from their housing tanks and assigned to specific experimental groups using a computerized random number generator ( e.g. , www.random.org). Both male and female zebrafish can be used for the experiments since no gender difference in writhing-like responses to acetic acid was observed in the reference paper. Note, however, that if using additional behavioral endpoints and/or treatments, animals from both sexes must be tested separately to avoid false positive or negative findings, and consistent with recent NIH guidelines on the inclusion of both males and females in biomedical experimentation. All animal experimentation should be performed in accordance with the Institutional Animal Care and Use Committee (IACUC) following national guidelines and standards.
Steps
ACCLIMATIZATION AND BASELINE BEHAVIORAL RECORDINGS ●TIMING ~ 1 h for acclimatization; 6 min per animal
Transport animals from their holding facility to the experimental room for acclimation 1 h prior to the experiments. Avoid low- or high density of fish to prevent social isolation or crowding stress (tanks must have 1–2 fish per 1 L of water). Fish must acclimate to the facility before testing, and the water used must have similar physicochemical characteristics to those of housing tanks. The use of home tank or holding tank water (with properly adjusted temperature and salinity) is required. !CAUTION: Ensure that the experimental tank is filled with non-chlorinated water before use set at optimal temperature (27-28°C).
Mount a camera on the frontal side of tank test and connect video recorder to the power in a switch-on mode. Provide adequate illumination (fluorescent light bulbs), to ensure that the enlightenment is proper to differentiate the subject from the apparatus, allowing a precise detection of fish. However, avoid excessive brightly lit environments (stressful for zebrafish) and dark areas (poor fish recognition and video-tracking detection of locomotion if desirable). ? TROUBLESHOOTING
Prepare the acetic acid solutions in 1.5 mL microcentrifuge tubes (2.5% or 5.0% diluted in PBS or 0.9% NaCl).
Transfer the fish from the holding tank to the experimental tank and start recording for 6 minutes to measure the baseline behavioral phenotypes. Animals should be transferred individually to the experimental tank. Ensure adequate transport to minimize handling stress. !CAUTION: If more than a single injection is applied, the baseline behavior should be recorded after the first injection to avoid false positive/negative results. ?TROUBLESHOOTING
INTRAPERITONEAL INJECTIONS ●TIMING ~ 3 min per animal
Before i.p. injections, video recordings must be stopped.
Remove the fish from the tank with a fishing net and proceed with the i.p. injection using a BD Ultra-fine™ 30U syringe (needle size 6 x 0.25 mm) with a volume of 10 μL (which does not impair normal zebrafish behaviors). Animals must be gently handled, briefly anesthetized, and later immobilized using a small wet fishing net (< 5 s). The i.p. injections should be quickly performed into the midline between the pelvic fins. To minimize potential interference of drugs in the behavioral endpoints measured and to allow a fast evaluation of the swimming activity, avoid using anesthetics (tricaine or other similar drugs). Injections can be quickly performed through the net in fish previously anesthetized in cold water without affecting complex behaviors ( e.g. , depth preference, immobility, and locomotion). ?TROUBLESHOOTING
FINAL BEHAVIOR RECORDING ●TIMING 6 min per animal
Relocate the fish into the experimental tank to record the behavioral phenotypes following i.p. injections for 6 minutes.
CLEANUP ●TIMING 1–2 min per animal
Stop the behavioral recording and remove the fish from the experimental tank. Animals must be euthanized following national guidelines and standard protocols.
Discard the water used previously, wash the tank thoroughly with tap water and refill it with non-chlorinated water before testing another fish.
Repeat steps 4–9 for the next subject tested.
VIDEO ANALYSES ●TIMING 20–30 min per animal
Digital pictures of fish at sagittal plane (Fig. 2) must be taken every 30 s, totaling 24 photos per fish. We strongly recommend the use of "Prt Sc" ( print screen ) Windows tool.
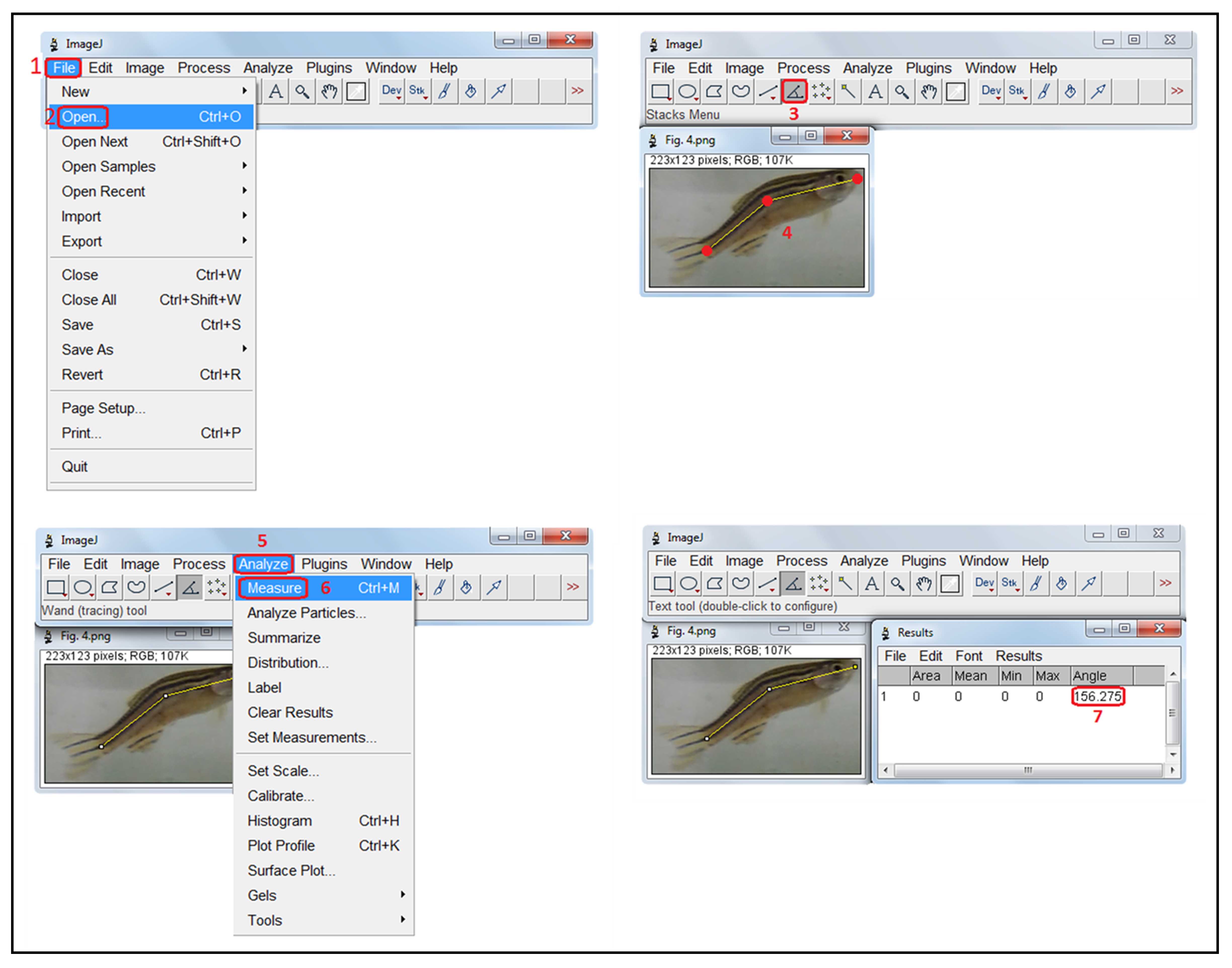
With ImageJ software (available for download at https://imagej.nih.gov/ij/download.html), open the digital pictures of fish sagittal plane that represent the first 30 s (1-2) , and using the angle tool (3) , select three positions to estimate the fish body angulation: a frontal (in the front of the head), a central (in the middle of the animal’s body – between the anal and dorsal fins), and a posterior one (at the caudal fin) (4) . Then, analyze and measure the fish angular value (5-7) . !CAUTION: Results must be subtracted from 180° to calculate a value representing the body curvature index (Fig. 3) . ?TROUBLESHOOTING
STATISTICAL ANALYSES ●TIMING 20–30 min, depending on amount of data collected
Several options exist for analyzing the data, based on the specific experimental design. Use the nonparametric Wilcoxon-Mann-Whitney U-test or parametric Student’s t -test (if data are homoscedastic or normally distributed) for comparing two groups. For more than two groups, use analysis of variance (ANOVA), followed by appropriate post-hoc comparison ( e.g. , Tukey, Dunn, Student-Newman-Keuls or Dunnet tests). Depending on the study design, an n -way ANOVA can generally be applied to assess various factors ( e.g. , drug/treatment, dose, sex, strain, time, trial, age). Even if a small number of cohorts is used ( n = 5), robust differences can be detected in acetic acid-treated fish vs. PBS (control group) ( e.g. , effect size calculated using Cohen's d = 3.646). Thus, the use of adult zebrafish to assess pain-like responses following a single i.p. acetic acid injection brings direct “3R's” benefits (refinement, replacement, reduction) of animal experimentation. Use ANOVA with repeated measures to assess time-dependent modulation of the observed phenotypes, if necessary. To facilitate data analysis, export data from every 30 s into separate Excel spreadsheets (one spreadsheet per fish tested in a 6-min trial). The area under curve (AUC) from each individual can be further calculated to express the specific behavioral endpoint using specific statistical packages ( e.g. , GraphPad Prism).
ANTICIPATED RESULTS
The anticipated results described below show robust pain-like responses in zebrafish as reported elsewhere.





