Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assays Using Broth Microdilution Method
Tomasz Swebocki, Alexandre Barras, Aleksandra Maria Kocot, magdalena.plotka, rabah.boukherroub
Disclaimer
The protocol provided herein is intended for informational purposes only. The authors of this protocol have made reasonable efforts to ensure the accuracy and reliability of the information presented. However, the authors do not assume any responsibility for any accidents, injuries, or damages that may occur as a result of following the protocol.
Experimentation and scientific procedures involve inherent risks, and it is essential to exercise caution, adhere to safety guidelines, and consult with qualified professionals when necessary. The authors cannot be held liable for any direct, indirect, incidental, consequential, or special damages arising out of the use of this protocol.
By utilizing this protocol, you acknowledge and accept the risks associated with experimentation and agree that the authors shall not be held responsible for any unfavorable outcomes. It is your responsibility to assess the suitability of the protocol for your specific needs and take appropriate safety measures to ensure the well-being of yourself and others involved.
Remember, safety should always be the highest priority when conducting any experiments or scientific endeavors.
Abstract
The presented protocol outlines a comprehensive assessment of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values for bacterial cell cultures. These experiments are vital for screening bacterial susceptibility to antibiotics and substances with potential antibacterial properties. The protocol not only covers the necessary preparatory steps but also introduces the application of the widely recognized Gompertz model. The protocol ensures a smooth execution of the assessment through thorough preparation and step-by-step instructions. The user-friendly instructions provided enable researchers to easily follow the protocol, facilitating the implementation of the assessment. By adhering to the outlined procedures, researchers can acquire a deeper understanding of bacterial susceptibility, evaluate the efficacy of antimicrobial agents through MIC and MBC values, and contribute to the advancement of antibacterial strategies.
Before start
Be sure to read the protocol fully, before starting any manipulation. Be sure that all the equipment needed is working properly and you have enough time to do all the manipulations. If it is your first time doing this, be sure to plan more time, as usually first times take longer. Be sure that both you and environment is well protected and your laboratory regulations allow for manipulation with bacteria.
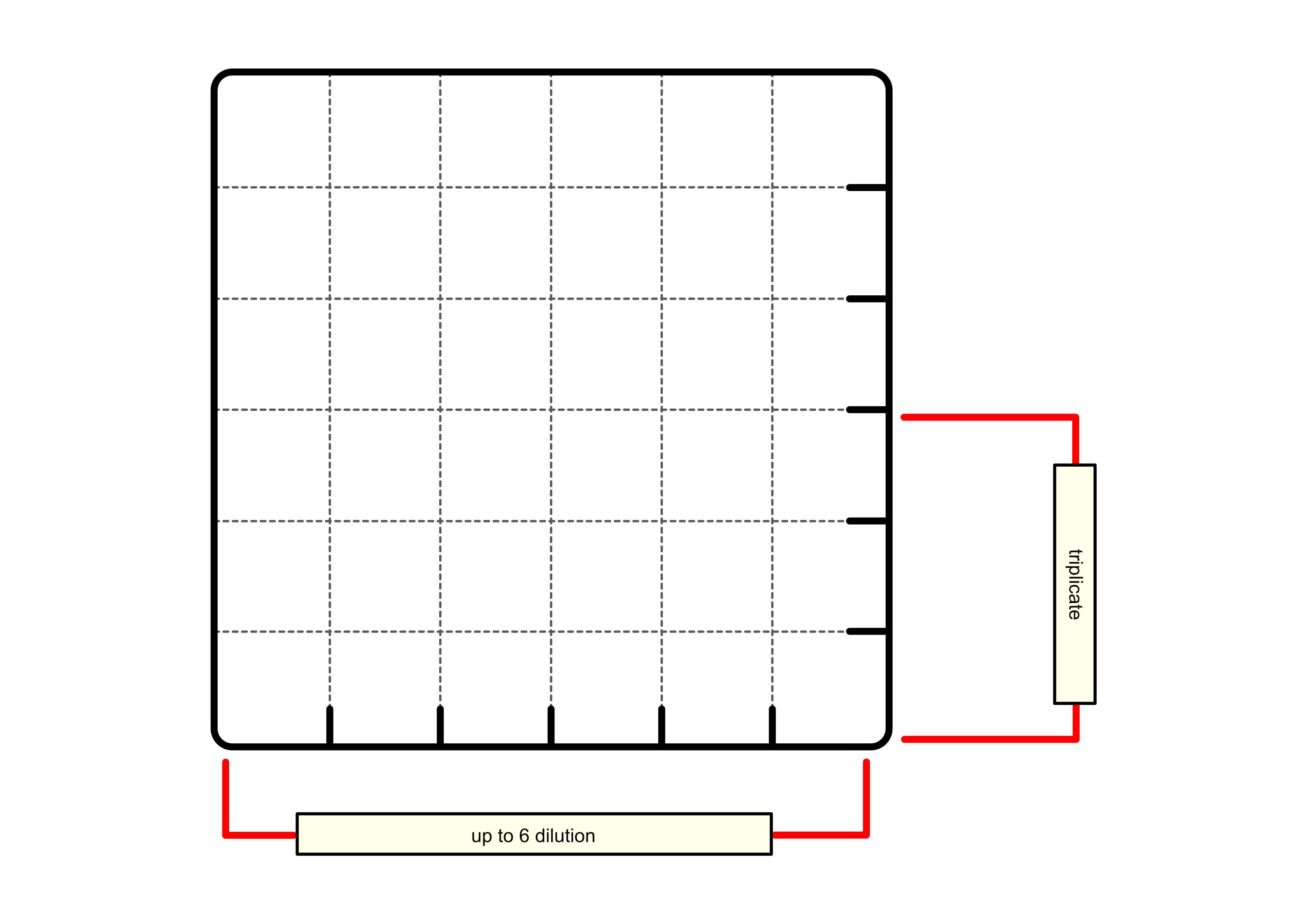
For a single test, that consist of a triplicate you will need around 4 mL of MHB, however, it is recommended to have much more prepared (preferably around 20 mL) in case any mishaps occur. You have to have an inoculated medium on a Petri dish with discrete colonies already prepared.
Steps
Pre-preparation of stock colony
Take the inoculated Petri dish and transfer one colony to a tube with approx.3mL of fresh MHB .
Incubate the tube for around 3h 0m 0s (or more, depending on the strain and the size and age of the colony used) at 37°C.
Preparation of diluted standardized inoculum
- Is greater than 0.1, dilute the culture with MHB to reach a value of 0.1,
- Is between 0.09 and 0.1 you can move to a next step,
- is below 0.09 put the culture back and incubate it for 15-30 minutes more.
Pour 10mL of MHB into a trough and add 100µL of the standardized inoculum . Mix it by flushing couple of times with a pipette.
Preparation of 96-well microplate
Dissolve tested substance ( X ) in MHB at twice the maximum concentration for the test.
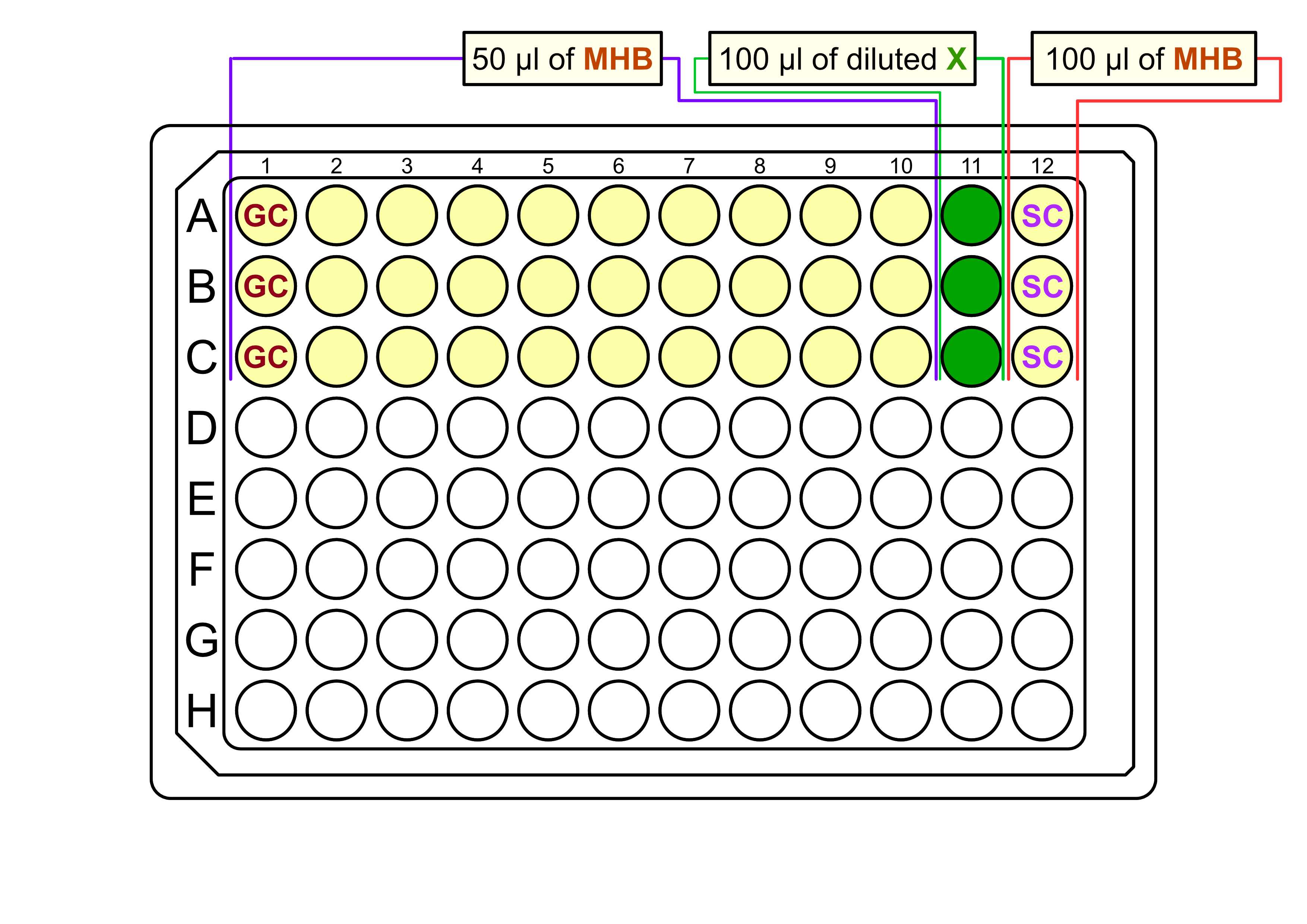
Pour around 10mL of MHB to the trough and:
- Add
50µLof MHB to each well in columns 1-10 (growth control, GC ( 1 )+ serial dilution ( 2-10 )), - Leave wells in column 11 empty empty (highest concentration of serial dilution),
- Add
100µLto the wells in column 12 (sterility control, SC ).
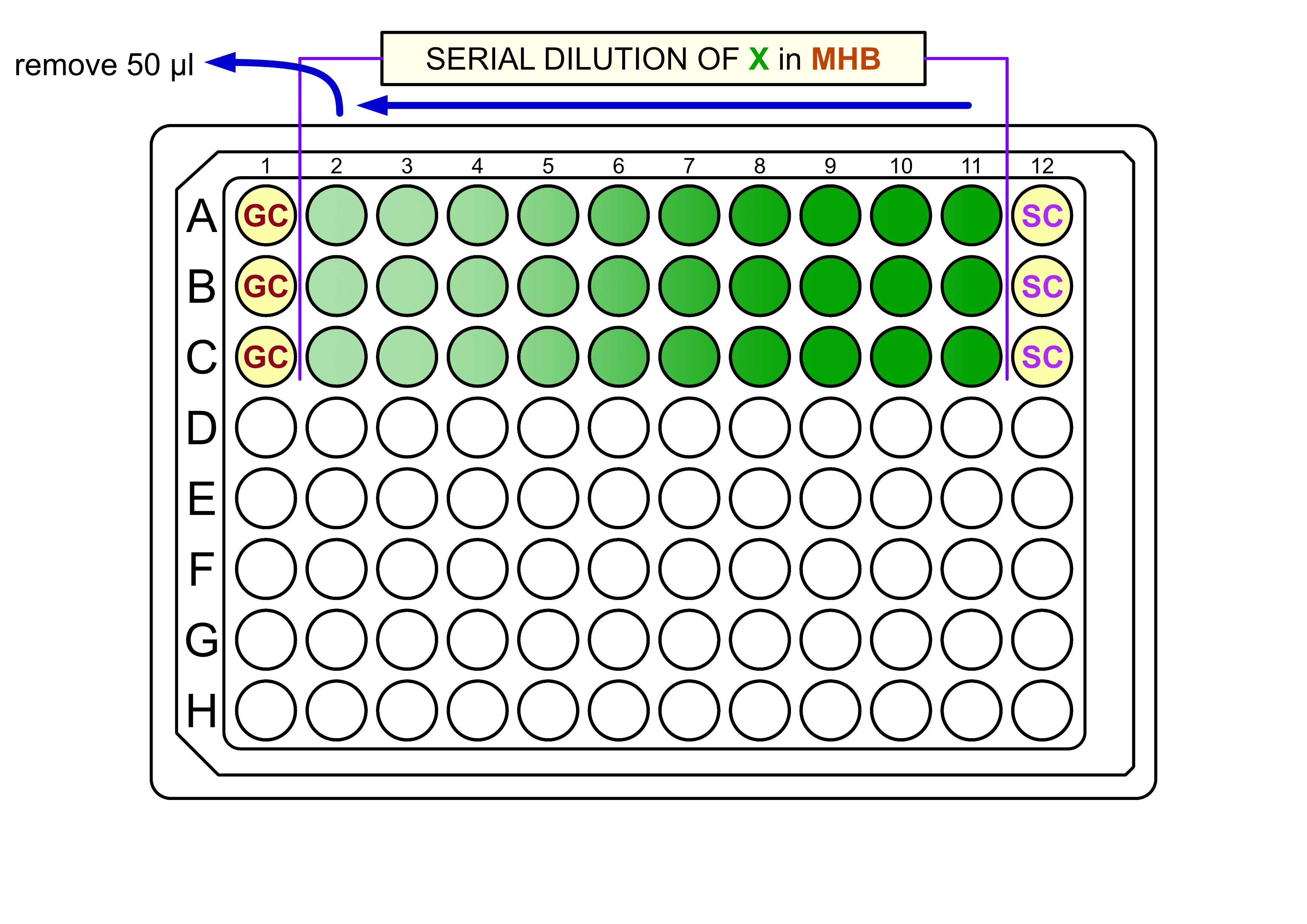
Add 100µL of the just prepared solution of X diluted in MHB to the wells in column 11 and remove and pass 50µL to next well until reaching wells in column 2 ,
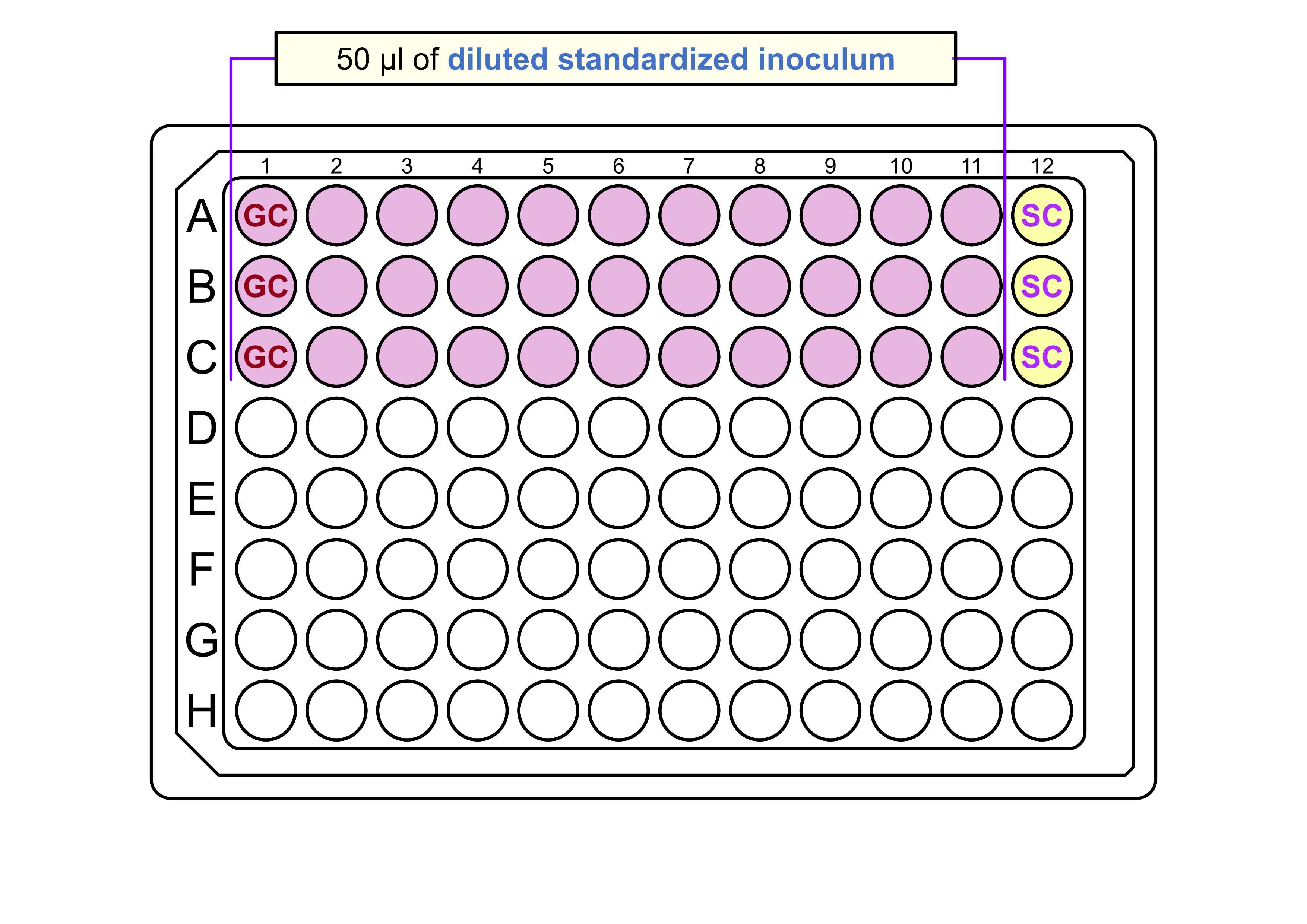
Using multichannel pipette add 50µL of diluted standardized inoculum to each well of columns 1-11 . For more information .
Put a protective film on that-prepared well plate. Cover it with a lid. Put a name and/or other details on the side of the plate. Avoid leaving any marks on the lid.
Incubate the plate at 37°C 0h 15m 0s .
Minimal inhibitory concentration (MIC) assessment
After the incubation take the microplate out of the incubator. Remove the lid from the plate and protective film if applied and let it cool down in Room temperature for 0h 15m 0s.
After that time put the microplate into the plate reader and read OD600 values of the wells' content.
Gather the data, and using plotting program such as Prism, OriginPro, Excel or Kaleida plot the data and fit it using modified Gompertz model.
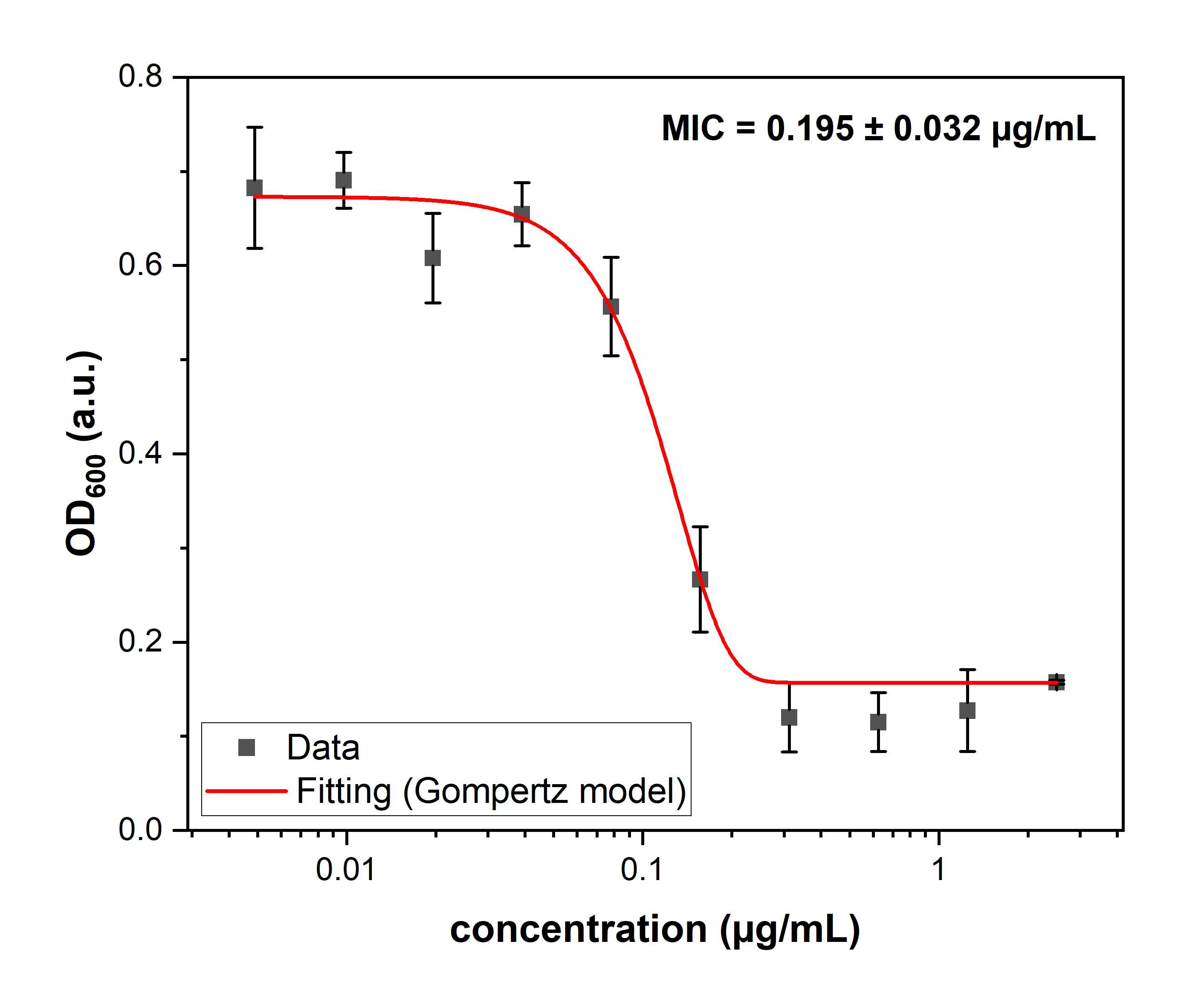
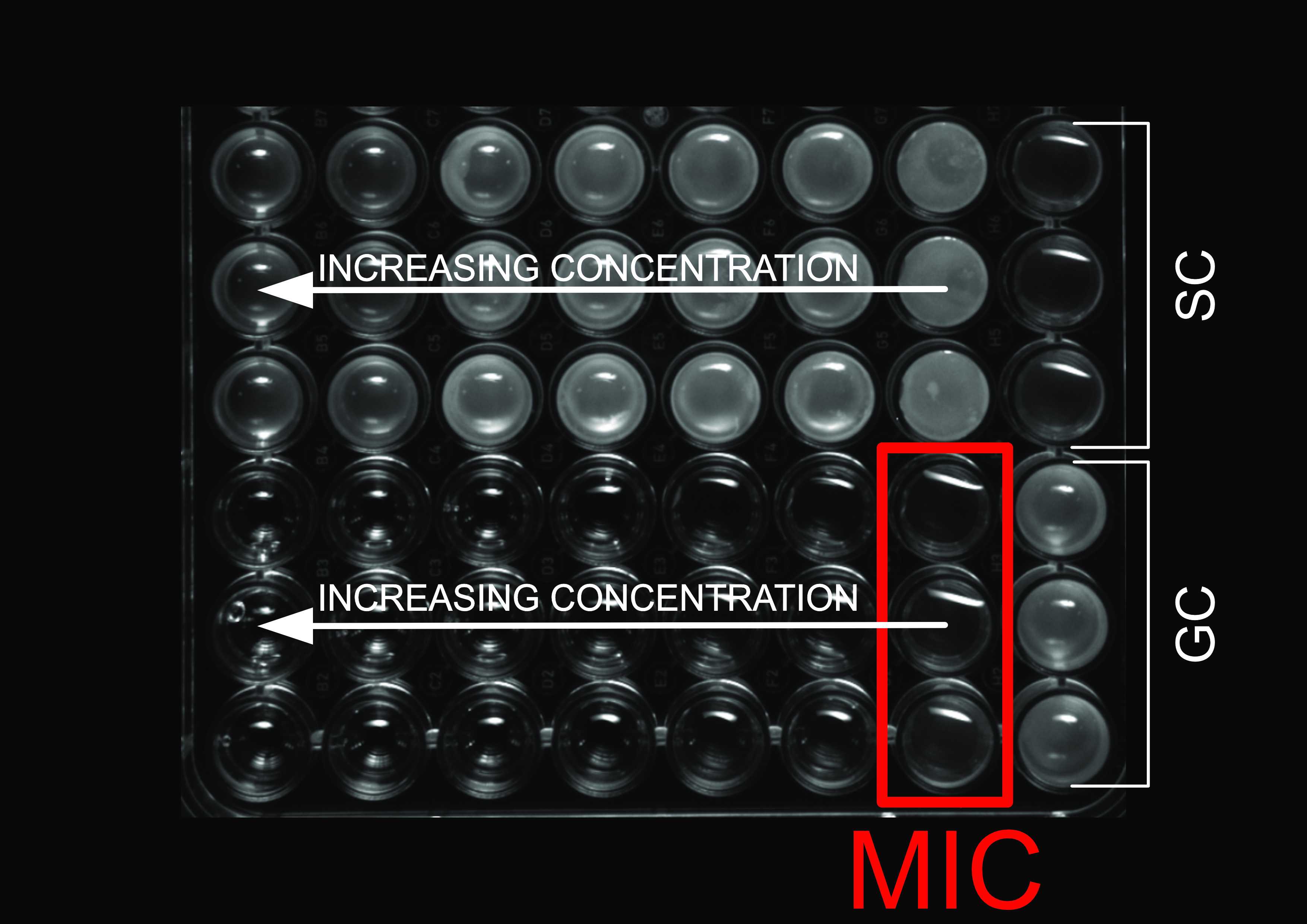
Minimal bactericidal concentration (MBC) assessment
Transfer 100µL of the content of the wells were no turbidity was observed to a separate 96-well microplate and dilute it ten fold serially with the solution of NaCl 9g/L
Next morning count the colonies on the plates.