MarineSediment-BlueC-eDNA
Marc Anglès d'Auriac, Kasper Hancke, Hege Gundersen, Helene Frigstad, Gunhild Borgersen
Abstract
This protocol was established for the Nordic Blue Carbon Project (2017–2020) for tracking the presence of kelp DNA in marine sediments, providing preliminary quantitative information on the original kelp biomass.
Nordic blue forests are coastal vegetated habitats, such as kelp forests, eelgrass meadows and rockweed beds, that are important natural sinks for carbon and thereby climate regulation. They also play an important role in climate adaptation. Simultaneously, blue forests are at high risk from climate change and other human impacts, such as eutrophication and coastal development.
The advent of eDNA methods for tracking animal or vegetal organisms in water or sediment matrices, has enabled not only geographical detection without collection of the organism but also temporal study of their presence in dated sediment cores. The fate of eDNA and its persistence in the environment is intimately related to its biophysical surroundings. eDNA entrapped in sediments may be preserved on long time scales, up to centuries, enabling historical tracking of the presence of a species on a site. The present protocol was developed for tracking eDNA of a predominant Norwegian kelp species, Laminaria hyperborea , in dated marine sediment cores. This approach enables confirmation of species specific contribution to carbon sequestration in an effort to evaluate marine sediments operating as carbon sinks.
This work was financed by the Norwegian Environment Agency (Miljødirektoratet) contract #17080044 and published in a NIVA report ISBN 978-82-577-7384-7, Norwegian Environment Agency Report M-2090|2021, Blue Carbon eDNA – A novel eDNA method to trace macroalgae carbon in marine sediments.
Steps
Core sampling
Equipment assembly (Gravity corer)
- Add the necessary number of lead weights at the main rack.
- Mount the supporting frame and secure it by fastening the bolt.
- Mount the unit with the top lid. Open the fastening device and push the tube with the mechanical stop into the bottom. Lock the handles and secure with a bolt.
- Attach the carver and fasten with two nails.

Core field sampling
Lower the gravity corer into the sea and towards the sea floor at a controlled speed until it reaches 10m above the sea floor. Let the corer freefall the last 10m, and the corer penetrates the sediments by gravity A top lid is released when the corer hits the sea floor in high speed and close off the top of the corer. Upon retrieval of the corer, a vacuum is then created that retain the sediment core inside the corer during retrieval, given that the corer is handled with caution when exiting the sea water and entering the vessel. This is crucial to avoid the sediment from falling out, especially with coarser sediment types such as sand.

When the corer enters the vessel: remove the carver (nose piece), seal the bottom of the internal PVC tube with a tight-fitting plastic cap and secure it with waterproof tape. Remove the internal tube carefully from the corer in an upright position in order to keep the sediment-water interface intact. Cap the sediment with sea water from the sampling at the top and seal it with a plastic cap and secure it with waterproof tape. Take care to avoid air being trapped between the sea water and the cap.


In order to reduce the risk of sample contamination, the corer, nose piece and other equipment must be washed between every core cast, either with fresh water (preferrably) or with running sea water from a medium pressure hose on deck, as was done in the current project. The internal PVC tube that collects the core is new for every cast.
Equipment required on vessel for core sampling
Sediment cores were sampled at four sites in Frohavet, Trøndelag (NW coast of Norway, 63-64 °N ). Water depth ranged from 242 to 531 m and the sediment type was silt and very fine sand. The sampled core length ranged from 61 to 121 cm.
Sediment corer: KC Denmark Gravity Corer. The corer is an open tube fitted with a weight so that gravity can force it sufficiently deep into the sediment in order to retrieve a sediment core. The KC Denmark Gravity Corer recovers an 88.9 mm diameter core of undisturbed sediment. The corer is designed with several detachable core tubes each with a length of 150 cm. The corer is made from stainless steel and consists of a corer body with 6 steering fins and a weight platform, steel corer, internal PVC liners (preferably transparent), lead weights, orange peel closing system (“core catcher”) and a carver with cutting edge (nose piece).
Equipment
| Value | Label |
|---|---|
| Gravity corer | NAME |
| KC Denmark | BRAND |
| 13.540 | SKU |
| The 13.540 gravity corer Ø101,6 mm is designed for taking samples in sandy sediment featuring a detachable core tube with a maximum length of 6 m, (optional). Up to 12 m in soft or muddy sediment. The corer is fully manufactured from AISI 316 stainless steel. Finish: Electro polish. | SPECIFICATIONS |


Other required equipment include: nails, hammer, cutting tools, ruler, PVC tape/gaff tape, waterproof writing utensils and safety equipment. The vessel must be equipped with a navigation system and being able to position the boat steadily during sampling.
Core sample transportation and storage
Photograph each core in order to record basic characteristics of sedimentary layers while the sediment is fresh, to be able to identify any major disturbances during transport. Transport and store the cores standing up and maintained in their PVC tubes until opening in the laboratory. Storage until processing: in the dark at 4°C.



Core sample aliquoting
Preparation of whole sediment core for slicing
Open the PVC tube was at both ends and place it on top of a plunger fitting the inner diameter of the tube. Push the tube slowly and carefully downward until all the overlying seawater spills over the top and the sediment surface is even with the top of the tube. Push the tube further down so that a 1 cm thick section of the sediment core can be sliced off with a clean steel plate (slicer). Slice each core into 1 cm thick sections, until the desired depth (50 cm in this project and 12 sections were chosen for further analysis: 0–1, 2–3, 4–5, 6–7, 8–9, 10–11, 13–14, 17–18, 22–23, 28–29, 35–36, 43–44 cm depth layers).

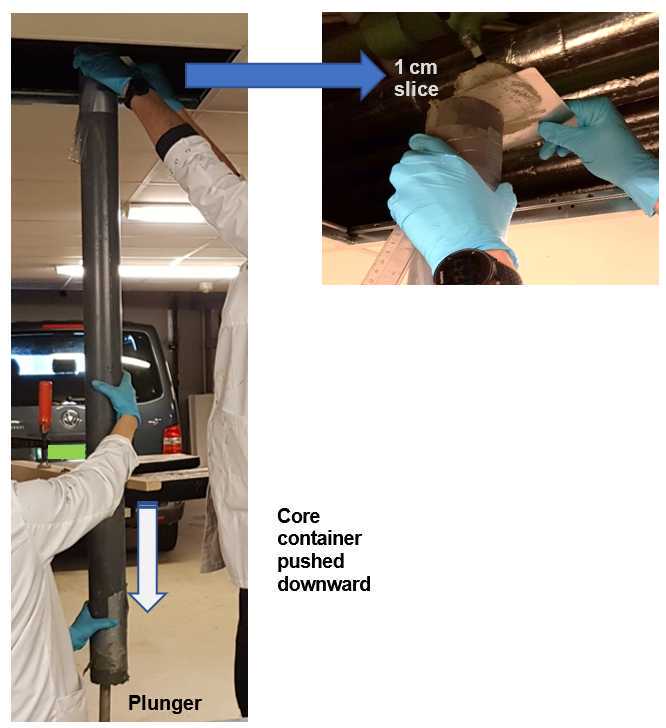
Individual sediment core slice aliquoting
Portion each 1 cm slice was into equal parts (i.e. aliquote) for different analyses. Rinse the equipment with

eDNA analysis
Laminaria hyperborea reference sample and core sample DNA extraction
DNA extraction from the core samples as well as from the kelp reference material was performed using
L. hyperborea reference material collection and preservation
Pure reference material was collected from living tangle and sugar kelp biomass near the city of Ålesund at the following geographic positions: 62°42'35.9"N 6°20'33.3"E on the 12/10/2018. Collected material was preserved dry in silica beads.

Laminaria hyperborea specific primers & qPCR protocol
| A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|
| Target | Species specificity | Primer name | Primers (Forward & Reverse) | Ta. oC | Product (bp) | Reference |
| cytochrome oxidase subunit-1 (COI) | Laminaria hyperborea | L_hyper_coi471F20 | CTCCCGGTATGACAATGGAT | 62 | 88 | This protocol and associated report |
| L_hyper_coi538R21 | AAAACAGGAAGCGATAACAGT |
Laminaria hyperborea specific primers
Mastermix:
+4.5µL
+0.75µL@ 10micromolar (µM) =500nanomolar (nM)
+7.5µL
+1.5µL
= 15.0µL
Equipment
| Value | Label |
|---|---|
| CFX96 Touch Real-Time PCR | NAME |
| qPCR | TYPE |
| Bio-Rad | BRAND |
| #1855195 | SKU |
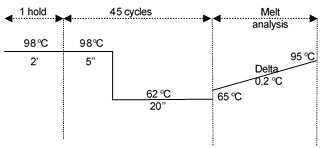
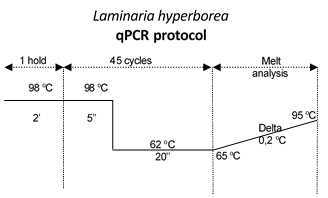
Laminaria hyperborea qPCR cycling conditionshttps://protocols-files.s3.amazonaws.com/files/dc57gkne.png?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIAIWSNCI5SNCPTWTQQ%2F20210415%2Fus-east-1%2Fs3%2Faws4_request&X-Amz-Date=20210415T153339Z&X-Amz-Expires=604800&X-Amz-SignedHeaders=host&X-Amz-Signature=9cd7ae13136500ac877a53340925ece0d0ad7c6cea3c8bbf01b904c9eb95a216
Laminaria hyperborea DNA reference material preparation