Manual Nanotrap Concentration and RNA Extraction for SARS-CoV-2 Viral Capture
Christine Moe, Matthew Cavallo, Stephen P Hilton, Jamie VanTassell, Julia Raymond, Julia Raymond, Marlene K Wolfe, Pengbo
Nanotrap
manual Nanotrap
wastewater
SARS-CoV-2
COVID-19
magnetic
magnetic virus particles
Ceres
nano
Abstract
This protocol details a method for SARS-CoV-2 capture and concentration through the use of Nanotrap® Magnetic Virus Particles from 40mL of wastewater sample.
Steps
Concentration Procedure
Place a 50 mL conical tube in a tube rack.
Vigorously shake the sample to re-suspend solids. Immediately move on to Step 3.
Add 50mL of wastewater to the conical tube inside the biosafety cabinet.
Transfer 40mL of the top supernatant into a new conical tube.
Add 10µL of BRSV to the conical tube.
Add 400µL of
Invert samples 3-4 times every 5 minutes at Room temperature for 0h 20m 0s.
#尊敬的用户,由于网络监管政策的限制,部分内容暂时无法在本网站直接浏览。我们已经为您准备了相关原始数据和链接,感谢您的理解与支持。
https://lh6.googleusercontent.com/fNX5vw9WfRxdeMswOTmK_XVOfrK2oIkGeqitKVq-69_asfGrRp7ZlrP_xHOzbEQUWXLoaVSBh3_8vIh0U2V6nxYqxCwvVCZTHJhKpPDbTlRVNz7Mld8UgIVN08nv3V-oXiNGlkgN
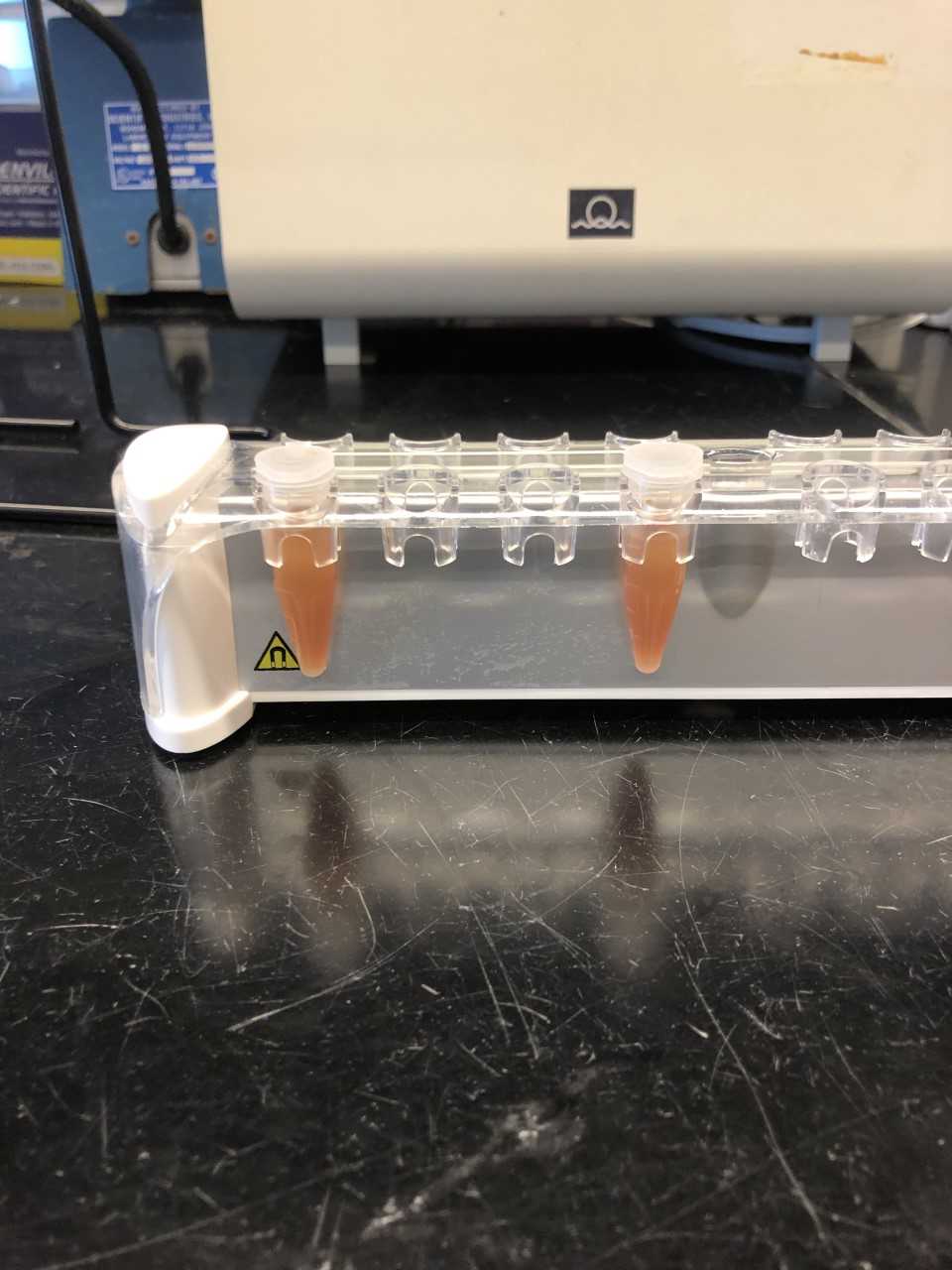
Use a pipette to discard the supernatant. Be careful not to disturb the red pellet of Nanotrap particles.
Add 1mL of
Transfer liquid to a 1.7mL tube. Place tubes on magnetic rack and allow to sit for 0h 2m 0s.
#尊敬的用户,由于网络监管政策的限制,部分内容暂时无法在本网站直接浏览。我们已经为您准备了相关原始数据和链接,感谢您的理解与支持。
https://lh3.googleusercontent.com/LWTsimwkEW337kul39KzsQlFzUEl45jLPRwWkgqUwCEOpwCnuovZZPjeFJ9oiBsQ1o2-av_0pk6x--3O-FYITS-6GKQn6JVSnTAUe6wFA0fOZ8yEoHFenXukIPnFSJ4ug38C0IHQ

Remove and discard the supernatant. Do not disturb the pellet.
Add 140µL of 1X PBS to the particle pellet.
In a separate 1.7 mL tube, add 560µL of 5.6µL of
In a regular, non-magnetic rack, add the Buffer AVL and carrier RNA to the particle pellet. Suspend the pellet by using the pipette to wash the sides of the conical tube with the lysis buffer, carrier RNA, and 1X PBS mixture.
Incubate the sample at Room temperature for 0h 10m 0s.
Use a magnetic rack to separate the magnetic Nanotrap particles from the sample. Allow sample to sit on the rack for 0h 2m 0s.
Transfer the supernatant to a 1.7 mL tube and discard the pellet.
Extraction Step (Qiagen Viral RNA Minikit)
Add 560µL of 96%
Add 630µL of the sample mix onto a QIAamp Mini column.
Centrifuge sample at full speed for 0h 1m 0s. Once complete, discard the filtrate.
Repeat Step 26 until all of the sample has gone through the column.
Transfer the QIAamp Mini Column into a new collection tube, and discard the tube containing the filtrate.
Open the QIAamp Mini Column and add 500µL of 0h 1m 0s. Once complete, discard the filtrate.
Open the QIAamp Mini Column and add 500µL of 0h 3m 0s. Once complete, discard the filtrate.
Place the QIAamp Mini Column into a new collection tube and discard the old one containing filtrate. Centrifuge the sample at full speed for 0h 1m 0s to remove any buffer AW2 carryover.
Place the QIAamp Mini Column into a final, labeled tube and discard the old one containing the filtrate
Open the QIAamp Mini Column and add 60µL of Room temperature for 0h 3m 0s.
Centrifuge the sample at 16000rpm,0h 0m 0s for 0h 2m 0s.
Separate sample into at least two aliquots. Store in -80°C freezer until it is ready for PCR.
Appendix I: Preparing Buffer Solution (1X PBS)
Begin making the 10X PBS Solution by mixing the following:
80gNaCl2gKCl14.4gNa2HPO42.4gKH2PO4800mLDdwater
Adjust the solution to 7.4 by adding 5% NaOH and/or 5% HCl.
Add more 1000mL.
Dilute the PBS solution by adding 100mL of 10X PBS to 900mL distilled water to create 1X PBS.
Dispense mixture into aliquots and autoclave for 0h 20m 0s. Store at room temperature.