MAIT Cell Adoptive Transfer
Liyen Loh, Marios Koutsakos, Katherine Kedzierska, Timothy S C Hinks, Bonnie van Wilgenburg, Huimeng Wang, Alexandra J. Corbett, Zhenjun Chen
Abstract
This is part 3.2 of the "Study of MAIT Cell Activation in Viral Infections In Vivo" collection of protocols.
Collection Abstract: MAIT cells are abundant, highly evolutionarily conserved innate-like lymphocytes expressing a semi-invariant T cell receptor (TCR), which recognizes microbially derived small intermediate molecules from the riboflavin biosynthetic pathway. However, in addition to their TCR-mediated functions they can also be activated in a TCR-independent manner via cytokines including IL-12, -15, -18, and type I interferon. Emerging data suggest that they are expanded and activated by a range of viral infections, and significantly that they can contribute to a protective anti-viral response. Here we describe methods used to investigate these anti-viral functions in vivo in murine models. To overcome the technical challenge that MAIT cells are rare in specific pathogen-free laboratory mice, we describe how pulmonary MAIT cells can be expanded using intranasal bacterial infection or a combination of synthetic MAIT cell antigen and TLR agonists. We also describe protocols for adoptive transfer of MAIT cells, methods for lung homogenization for plaque assays, and surface and intracellular cytokine staining to determine MAIT cell activation.
Before start
As MAIT cells are to be used for adoptive transfer, all procedures should be performed in a BSCII biosafety cabinet . All tools and reagents should be sterile.
Attachments
Steps
7 days or more after intranasal infection with S. Typhimurium, MAIT cells can be harvested ( see Note 9 ).
Prewarm collagenase media and shaking incubator to 37°C.
Mice should be euthanized (e.g., using a rising concentration of CO2 with a second method to confirm death).
Open the diaphragm by cutting the rib cage to expose both the heart and lungs. Gently perfuse the right ventricle with 8mL –10mL to dispense circulating blood. Perfuse using a 10-mL syringe and a 26-G needle. Efficient perfusion will result in lung inflation and a color change to pink/white.
Remove lungs using scissors to cut through the hilum and place into a 24-well plate containing ice-cold RPMI to transfer organs to the laboratory.
Chop lungs into fine pieces ( see Note 10 ).
Place lung tissue into a 1-mL Eppendorf tube containing 1–2 mL/lung of pre-warmed collagenase medium. Incubate tubes on their sides in a shaking incubator at 37°C, at 100rpm–180rpm, for 1h 30m 0s.
During this time prepare Percoll gradients and antibody cocktails ( see Table 1).
After 1h 30m 0s pour digested tissue through a 70-μm cell strainer and force through into a Petri dish with the plunger from a 1-mL syringe. Rinse residual sample with extra FACS buffer for maximum MAIT cell yield. Cells from multiple lungs (if required) ( see Note 11 ) are pooled into a single 50-mL Falcon tube with a total of 50mL.
Centrifuge at 400x g,0h 0m 0s for 0h 5m 0s to pellet the cells. Pour off supernatant (SN).
Resuspend cells in 20mL. Underneath this layer use a transfer pipette to layer 20mL ( see Note 12 ). Centrifuge this gradient at 800x g with the centrifuge brake OFF. Lymphocytes and other immune cells will form a visible interphase layer between the 40% and 70% Percoll post centrifugation.
During this centrifugation step, prepare single color controls. It is convenient to use part of a spleen forced through a 70-μm filter and resuspended in 5mL for 0h 5m 0s at 37°C, then washed once with 5mL.
Collect the interphase between 40% and 70% Percoll into a fresh 50 mL Falcon and top up with FACS buffer to a total of 50µL. Centrifuge at 400x g.
Pour off supernatant and resuspend in 5mL, transferring to a 10 mL Falcon tube. Centrifuge at 400x g.
Resuspend all lung cells in 750µL.
Block non-specific tetramer binding by adding 7.5µL, containing MR1-6- FP tetramer [8, 16] (no fluorochrome, 1:100). Incubate at Room temperature on a roller or bench rocker for 0h 15m 0s.
For lungs from 5 mice, add 750µL (Table 1).
Cover in aluminum foil to protect fluorochromes from light and shake on roller for 0h 30m 0s Room temperature.
Wash with 10µL. Centrifuge at 400x g. Pour off supernatant.
Wash again with 10mL. Centrifuge at 400x g. Pour off supernatant.
Resuspend cells in 2mL and filter through 40 μm filter into non-pyrogenic FACS tubes.
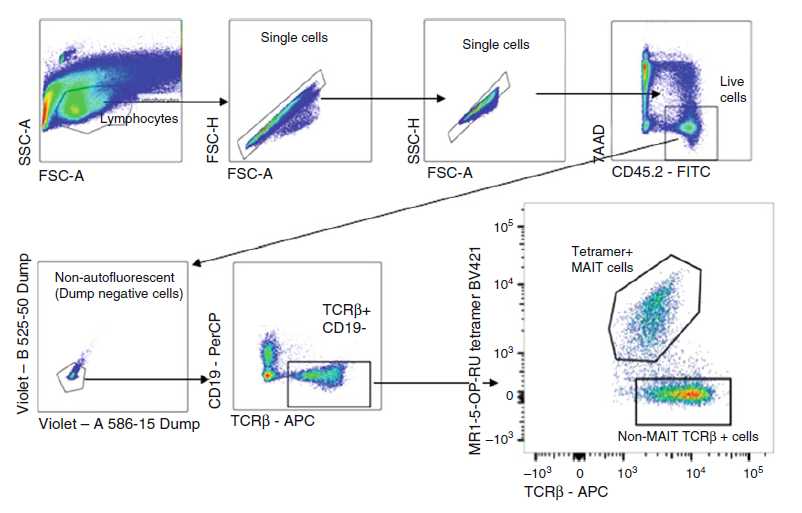
Sort live MAIT cells (defined as CD3+CD45+MR1-5-OP-RU tetramer+ cells) (Fig. 1) into 3mL in 15 mL Falcon tube. For detailed gating strategy, refer to [17]. Wash cells and adjust cell concentration to 5 × 105 cell/mL, allowing 105 in 200µL for injection to each mouse.
Inject 105 cells into the tail vein of recipient mice using cells suspended in 200µL in a 1-mL syringe with a 26-G cannula after warming the mice for 0h 5m 0s–0h 15m 0s with appropriate monitoring.
To deplete residual non-MAIT T cells ( see Note 13 ), inject recipient mice on days 2 and days 5 or 6 with 0.1mg each of purified anti-CD4 (GK1.5) and anti-CD8 (53.762) monoclonal antibodies i.v.
Rest mice for a total of 2 weeks post adoptive transfer to allow MAIT cell populations to settle in the host.

