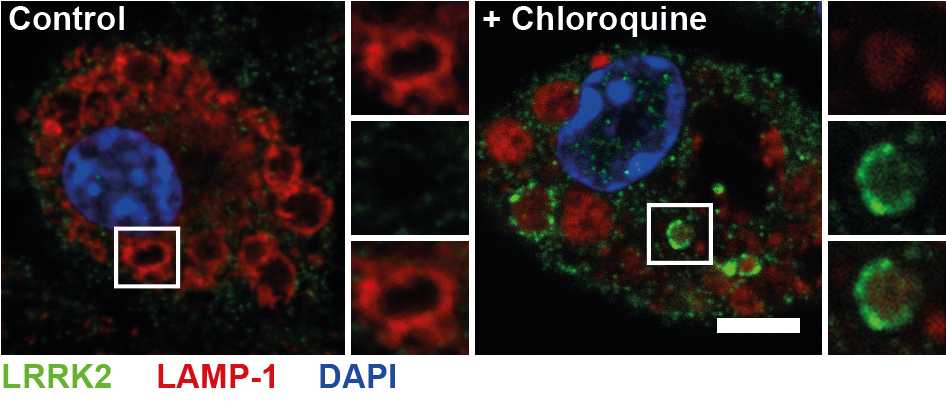
LRRK2 Immunofluorescent staining
sherbst
Abstract
Protocol for immunofluorescent staining for LRRK2 in cultured cells using the MJFF2 (c41-2) antibody.
Steps
Culture cells as usual on cover slips or in a plate suitable for imaging.
If using coverslips, we recommend Ø13 mm for cells cultured in 24-well plates.
Fix cells in 4 % PFA/PBS at 4°C for 0h 15m 0s.
Optional: at this step, coverslips or plates can be stored at 4 °C in PBS. Never allow samples to try out.
Permeabilise the plasma membrane by incubating samples in ice-cold MeOH for 0h 10m 0s. This can be done on the bench or the whole plate can be put into the -20 °C freezer. Make sure that samples are always submerged.
Wash samples once in PBS
Incubate samples in blocking buffer for 0h 20m 0s at Room temperature
Incubate samples in primary antibody solution ( MJFF2 (c41-2) antibody diluted 1:100 in blocking buffer) for 1h 0m 0s at Room temperature
Wash samples three times in PBS
Incubate samples in secondary antibody solution ( eg anti-rabbit-Alexa Fluor™ 488 diluted 1:800 in blocking buffer) for 0h 45m 0s at Room temperature in the dark
Wash samples twice in PBS
Incubate sample in DAPI staining solution for 0h 10m 0s at Room temperature in the dark
Wash samples twice in PBS
Mount coverslips onto slides using a mounting medium of choice (eg DAKO Fluorescence Mounting medium) and let try at RT in the dark.* If using well-plates, add PBS to plates.
-
Keep samples in the dark and store them at 4 °C for short-term storage.