KAPP-Sen TMC: Whole Pancreas Preparation
Cristina Aguayo-Mazzucato, Kanako Iwasaki
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
Whole pancreas and tissue blocks preparation
Steps
Overview
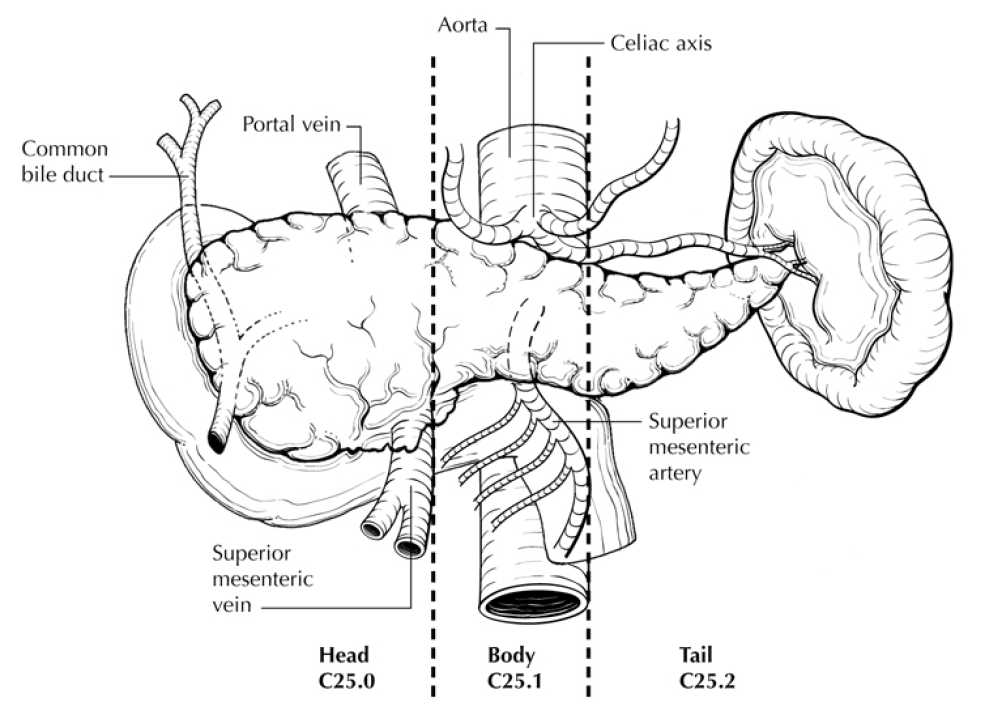
All the whole pancreases were procured by the University of Texas Health San Antonio and shipped to Joslin Diabetes Center, University of Harvard Medical . The pancreases were cut into the Head, Body, and Tail of the locations along the guideline (1), cut into 4mm thickness, and placed into order paraffin or frozen cassettes. The paraffin cassettes were fixed in 4% paraformaldehyde for 24 hours and processed for paraffin embedding at Joslin Histology Core . The frozen cassettes were molded with O.T.C. compound ( Fisher Scientific No.23-730-571 ) and frozen in the cold 2-Methylbutane ( Fisher Scientific No.60-048-072 ) bath followed by dried and stored at -80°C. At least three paraffin cassettes per each location of Head, Body, and Tail were shipped to The Jackson Laboratory ; all the procedures were done at 4°C and avoided exposure to direct light.
Preparation of Human Pancreas
30 minutes before the pancreas arrives:
1. Prepare 2-Methylbutane bath
-Fill the bottom of the plastic big beaker with dry ice (use tip, not powder dry ice)
-Place a glass beaker inside a larger plastic beaker
-Add dry ice around the glass beaker up to 400.
-Pour 100% EtOH onto dry ice until it appears "slushy" - keep adding dry ice chips and ethanol as needed. Should be bubbling.
-Fill the glass beaker about half-way with 2-Methylbutane and let it chill for 15-20 minutes before using.

2. Prepare Cassettes
-Name the cassette "JDC-WP-###-X" to both green paraffin cassettes and clear frozen mold. #### should be the experimental number. X should be alphabetical from a to z. After z, create aa, ab, up to ae.
-Add OCT to cover the bottom of a labeled frozen mold without bubbles.
-Set everything like the pictures

3. When the Pancreas Arrives
-Get ice and place between two trays.
-Pour PBS into the top tray until the pancreas is completely immersed.

4. Te st isopentane to make sure that it is cold enough - with long forceps, put mold with OCT medium into chilled isopentane (if it doesn't turn within 5 seconds, wait longer, and add more dry ice and more ethanol).
5. After removing fat from the pancreas, spread out pancreas on wax, and cut into Head, Body, and Tail (see Section " Examination of Specimens from Patients with Carcinoma of the Pancreas " for the definition of anatomical locations), and measure the weight.
-Paraffin: put it in the green cassettes from 'a'
-Frozen: put it onto the mold and add another OTC. Dry it by placing it on dry ice, and standing it up at an angle.

6. Be sure to bleach everything for over 30 minutes. Always treat it as contaminated with an infectious disease.

Examination of Specimens from Patients with Carcinoma of the Pancreas
From "Pancreas Exocrine 4.0.0.1" page 8, explanatory note B. Definition of Location