Isolation of bacteria and fungi from cheese rind microbiomes
Emily C.P. Weiss
Bacteria
bacterial
fungus
fungi
microbes
isolate
isolates
cheese
microbial community
community
contaminant
contamination
separate
single
colony
colonies
Abstract
This protocol describes how to harvest and stock bacterial and fungal isolates from cheese rind microbial community samples.
Steps
Harvesting the cheese rind biofilm
If you are starting from a glycerol stock of a previously stored rind community, proceed directly to the dilutions in step 8. If starting from an intact cheese, start at step 2.
Holding the cheese in one (gloved) hand and a sterile razor blade in the other, gently scrape the surface of the cheese to remove the rind biofilm. Avoid applying too much pressure, as you will start to dig into the paste. Scrape the rind biofilm into a weigh boat or other container to clean off the blade as needed.
After removing the desired amount of rind, use a sterile wooden dowel or a pipette tip to homogenize the harvested rind.
Resuspension and dilution
Add a lentil-sized amount of harvested cheese rind biofilm to 500 μL of sterile 1× phosphate-buffered saline (PBS) + 0.05% Tween in a 1.5 mL microcentrifuge tube.
Using a small sterile pestle, gently grind the cheese rind sample until it is completely resuspended in the buffer.
Equipment
| Value | Label |
|---|---|
| Disposable Pellet Pestles | NAME |
| Fisher Scientific | BRAND |
| 12-141-364 | SKU |
Add another 500 μL of PBS + 0.05% Tween to the suspension.
Vortex thoroughly to mix the suspension.
Make 10-fold serial dilutions of the cheese rind suspension eight times in PBS + 0.05% Tween to create 10-1, 10-2,, 10-3,, 10-4,, 10-5,, 10-6,, 10-7,, 10-8dilutions.
Bacterial isolation
Use four LB + 100 μg/mL cycloheximide agar plates to plate 100 μL each of the 10-5, 10-6, 10-7, 10-8dilutions from step 8.
Leave the plates at room temperature until you can see individual bacterial colonies on at least one of the dilution plates.
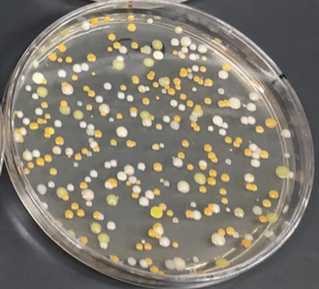
Using a sterile toothpick or other sterile instrument, gently touch a single individual colony and quadrant-streak onto a fresh LB + 100 μg/mL cycloheximide agar plate.
Leave at room temperature until single colonies appear.
Using a sterile toothpick or other sterile instrument, gently touch a single pure individual colony and inoculate into 2 mL LB broth.
Shake the culture at 200 rpm at room temperature until cloudy.
Add 250 μL of culture to 750 μL of sterile 80% glycerol in a labeled 2 mL cryotube, invert to mix well, and immediately store tube at −80 °C.
Repeat this process until all desired colonies from the original community plate have been stocked.
Fungal isolation
Use four PCAMS + 50 μg/mL chloramphenicol agar plates to plate 100 μL each of the 10-1, 10-2, 10-3, 10-4dilutions from step 8.
Leave the plates at room temperature until you can see individual fungal colonies on at least one of the dilution plates.
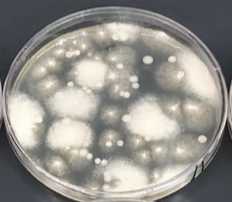
Using a sterile toothpick or other sterile instrument, gently touch a single individual colony and quadrant streak onto a fresh PCAMS + 50 μg/mL chloramphenicol agar plate.
Repeat as many times as needed onto a new plate to get a pure single colony.
From a pure single colony, use a sterile toothpick to pick up fungal spores and then streak the spores in horizontal lines back and forth across a new full plate. Then turn the plate 90° and streak in horizontal lines back and forth across the plate. The goal is to spread the fungus evenly over the surface of the plate.
Once the fungus is grown and sporulated (~5–7 days), add 1 mL of sterile PBS with 20% glycerol to the plate. Use a cell scraper to gently scrape the surface of the plate to collect the fungal spores into the liquid.
Use a 1 mL pipette to collect the liquid with the resuspended spores. Vortex to mix thoroughly.
Transfer the liquid to a labeled 2 mL cryotube and store at −80 °C.
Repeat this process until all desired colonies from the original community plate have been stocked.

