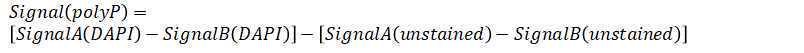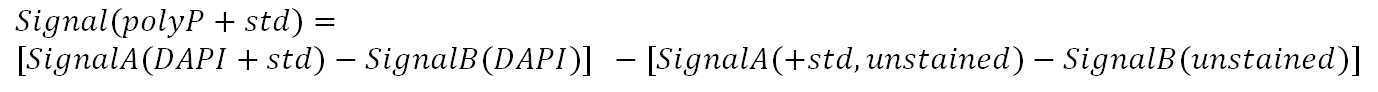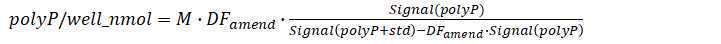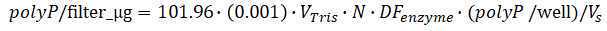Inorganic polyphosphate from microalgae: A DAPI-based estimation in microtiter plate
Ying-Yu Hu, Zoe V. Finkel
Abstract
The DAPI-based fluorometric estimation of polyphosphate from microalgae has been widely used in field samples since the method was published by Martin P. et al., where fluorescence of DAPI-stained samples is analyzed in quartz cuvettes by spectrofluorometer. In order to minimize the photobleaching of DAPI and reduce the consumption of reagent, time and labor, we have now scaled this method to 96-well black microtiter plate. Regarding to the matrix effects in microplate, the calculation has been modified accordingly.
Our method permits processing nine samples by using only 250 uL of extracted sample, 500 uL of RNase, 500 uL of DNase, 1000 uL of proteinase and <2000 uL of DAPI (100 uM). A lid with black film can protect all DAPI-stained samples from photobleaching.
Before start
Steps
Sample collection
Filter microalgae in liquid media onto precombusted GFF filters, using gentle vacuum pressure (5 inches Hg).
Equipment
| Value | Label |
|---|---|
| Filter forceps | NAME |
| blunt end, stainless steel | TYPE |
| Millipore | BRAND |
| XX6200006P | SKU |
| http://www.emdmillipore.com/ | LINK |
Rinse sample with filtered seawater
Place sample filters in cryogenic vials
Filter blank media (without cells) through precombusted GFF filter as blank.
Flash freeze filters and stored at -20°C
Freeze dry before measurement.
Equipment
| Value | Label |
|---|---|
| FreeZone® 2.5 L Benchtop Freeze Dryers | NAME |
| Labconco® | BRAND |
| 700202000 | SKU |
Preparation of reagents
Tris buffer 20mM 7.0
In a 1 L volumetric flask, top 20mL 1M 7.0 Tris buffer to 1 L with MilliQ
Filter through Rapid-flow and store at Room temperature
Equipment
| Value | Label |
|---|---|
| Sterile Disposable Filter Units with PES Membrane | NAME |
| Thermo Scientific™ Nalgene™ Rapid-Flow™ | BRAND |
| 5964520 | SKU |
| https://www.fishersci.com/us/en/home.html | LINK |
PolyP primary standard stock
Weigh one glass pellet of polyP (45) and write down the weight.
Equipment
| Value | Label |
|---|---|
| Microbalance | NAME |
| Cubis series | TYPE |
| Sartorius | BRAND |
| MSE6.6S-000-DM | SKU |
| https://www.fishersci.com/us/en/home.html | LINK |
Transfer the pellet into a 100 mL graduated cylinder.
Dilute to 100 mL with Tris 20mM 7.0
Aliquot primary stock into 10~50 uL per microtube with Stepper and store at -20°C
PolyP secondary standard stock
If the pellet is far more than 10 mg, dilute primary to secondary to bring down the concentration before preparing working standard
Proteinase K 20mg/ml
Add 25mL MilliQ directly into the original package of Proteinase K, vortex to mix
Aliquot 600 uL to microtubes (around 45 microtubes) and keep frozen at -20°C
DAPI primary stock 14.3mM
Add 2mL MilliQ directly into the original package and keep frozen at -20°C
Preliminary extraction efficiency test
Prepare boiling bath.
Equipment
| Value | Label |
|---|---|
| VWR® Advanced Hot Plates | NAME |
| VWR | BRAND |
| 97042-658 | SKU |
Equipment
| Value | Label |
|---|---|
| Hollow Polypropylene (PP) Ball Bath Covers, 20 mm | NAME |
| Cole-Parmer | BRAND |
| UZ-06821-04 | SKU |
| http://www.coleparmer.com | LINK |
Equipment
| Value | Label |
|---|---|
| Tube rack | NAME |
| Simport MultiRack™ | BRAND |
| CA48648-606 | SKU |
Transfer sample into glass centrifuge tube.
Equipment
| Value | Label |
|---|---|
| Disposable Glass Screw-Cap Centrifuge Tubes | NAME |
| 10 mL | TYPE |
| Corning® | BRAND |
| 99502-10 | SKU |
If the sample has less than 3 ug total particulate phosphate, use 2mL Tris Buffer 20mM 7.0 for each extraction.
Otherwise, use 4mL Tris Buffer 20mM 7.0 for each extraction.
Add 2mL or 4mL Tris buffer 20mM 7.0 , vortex and then sonicate.
Equipment
| Value | Label |
|---|---|
| Specific Pipette Tips 5mL | NAME |
| Thermo Scientific™ Finntip™ | BRAND |
| 21-377-304 | SKU |
| https://www.lifetechnologies.com | LINK |
Keep in boiling bath.
Sonicate
Vortex and then transfer extract to a 20 mL scintillation vial, label the vial with number of extraction.
Equipment
| Value | Label |
|---|---|
| Disposable Soda-Lime Glass Pasteur Pipets | NAME |
| 5 3/4" | TYPE |
| Fisherbrand | BRAND |
| 13-678-6A | SKU |
| https://www.fishersci.com/us/en/home.html | LINK |
Equipment
| Value | Label |
|---|---|
| VWR® Vials, Borosilicate Glass, with Phenolic Screw Cap | NAME |
| 22.18 mL | TYPE |
| VWR | BRAND |
| 66012-044 | SKU |
| 24-400 cap: VWR 89076-764 | SPECIFICATIONS |
Transfer 2 mL of extract to a 2 mL microtube.
13300rpm
Prepare DAPI working solution 100uM
Dilute 2.1µL of 14.3mM DAPI stock with 300µL MilliQ in a foil wrapped microtube and vortex.
Volume of total 100 uM DAPI = 30 X (10 X 3 X N + 3), where N is the number of culture samples tested.
In the dimmed room with only red light bulb on, by using either stepper or pipette, add 30µL 100uM DAPI to each sample in the plate.
Equipment
| Value | Label |
|---|---|
| Finntip™ Stepper Pipette Tips | NAME |
| 500 uL | TYPE |
| Thermo Scientific™ | BRAND |
| 9404170 | SKU |
| https://www.fishersci.com/us/en/home.html | LINK |
Equipment
| Value | Label |
|---|---|
| Finnpipette Stepper Pipette | NAME |
| Thermo Scientific™ | BRAND |
| 4540000 | SKU |
| https://www.fishersci.com/us/en/home.html | LINK |
Adhere black film on the top of a microplate lid and cover the plate with this lid.
Equipment
| Value | Label |
|---|---|
| Black Vinyl Films for Fluorescence and Photoprotection | NAME |
| VWR | BRAND |
| 89087-692 | SKU |
Shake at room temperature
Read fluorescence: excitation at 410 nm and emission at 550 nm
Equipment
| Value | Label |
|---|---|
| Varioskan LUX Multimode Microplate Reader | NAME |
| Thermo Fisher | BRAND |
| VL0L00D0 | SKU |
Plot fluorescence intensity versus number of extraction.
The number of extract (N) is the stationary point where the fluorescence of stained extract stops decreasing or the derivative of the fluorescence after that point is close to zero.
Extraction of polyphosphate from samples
Prepare boiling bath.
Prepare 37°C incubator.
Transfer samples into glass centrifuge tubes.
Add same amount of Tris buffer 20mM 7.0 as preliminary test, vortex and then sonicate
Place vials in boiling bath
Sonicate
Vortex and then remove extract to a 50 mL Falcon tube, and then .
Keep using the same pasteur pipet
Equipment
| Value | Label |
|---|---|
| Falcon® Centrifuge Tubes | NAME |
| Polypropylene, Sterile, 50 mL | TYPE |
| Corning® | BRAND |
| 352070 | SKU |
Combine extract 1~N into the same Falcon tube, keep extract N+1 in the centrifuge tube.
Enzyme treated extract
Centrifuge the mixture of 1~N extract3200rpm
Equipment
| Value | Label |
|---|---|
| General-purpose benchtop centrifuge | NAME |
| IEC CENTRA CL2 | TYPE |
| Thermo | BRAND |
| 00427 0F | SKU |
Transfer 4mL supernatant to a scintillation vial, add 40µL RNase and 40µL DNase
Incubate at 37°C, shake continuously
Equipment
| Value | Label |
|---|---|
| SHAKING INCUBATOR | NAME |
| 71L | TYPE |
| Corning® LSE™ | BRAND |
| 6753 | SKU |
Add 80µL Proteinase
Incubate at 37°C, shake continuously.
Enzyme treated N+1 extract
Centrifuge extract "N+1" (in the centrifuge tube) 3200rpm
Transfer 1.5mL supernatant into a 2 mL tube, add 15µL RNase and 15µL DNase
Incubate at 37°C, shake continuously
Add 30µL Proteinase
Incubate at 37°C, shake continuously
Enzyme treated standard amended extract
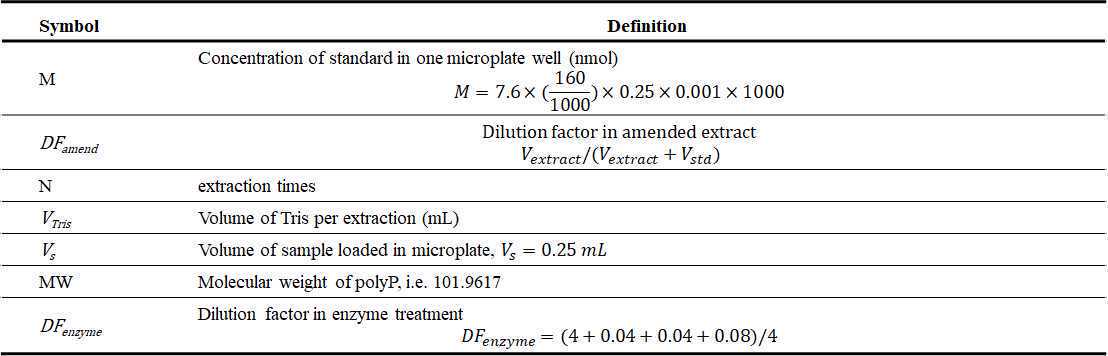
Prepare PolyP working standard 7.6uM
Based on the actual concentration of PolyP (45) primary or secondary standard stock, dilute a certain volume of stock with Tris buffer 20mM 7.0
For a final concentration 7.6uM
Total volume = 320 X N (ul)
N = sample number
Transfer 1680µL of enzyme treated extract into a scintillation vial.
Add 320µL 7.6uM polyP working standard to 1680µL of enzyme treated extract, vortex.
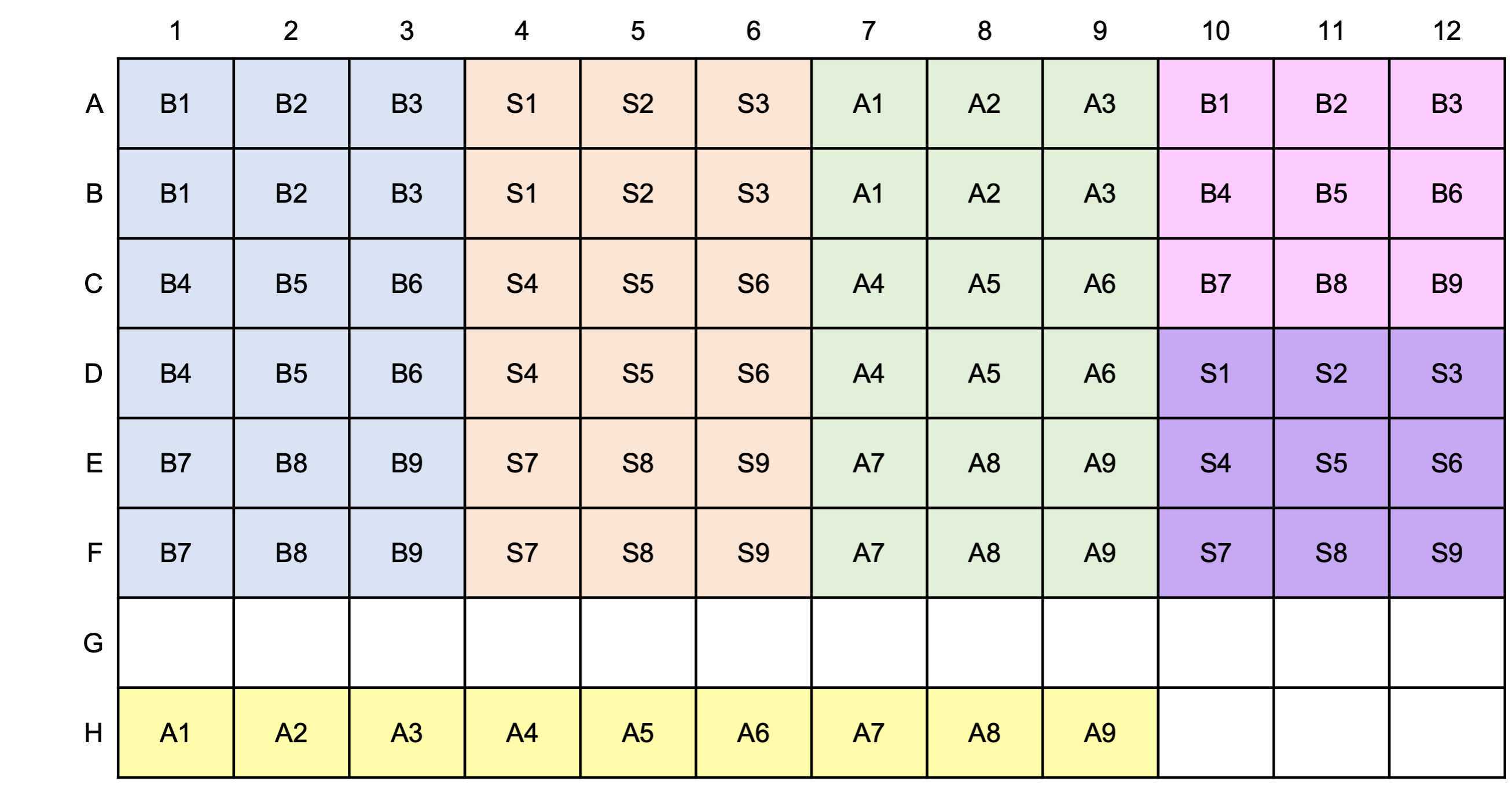
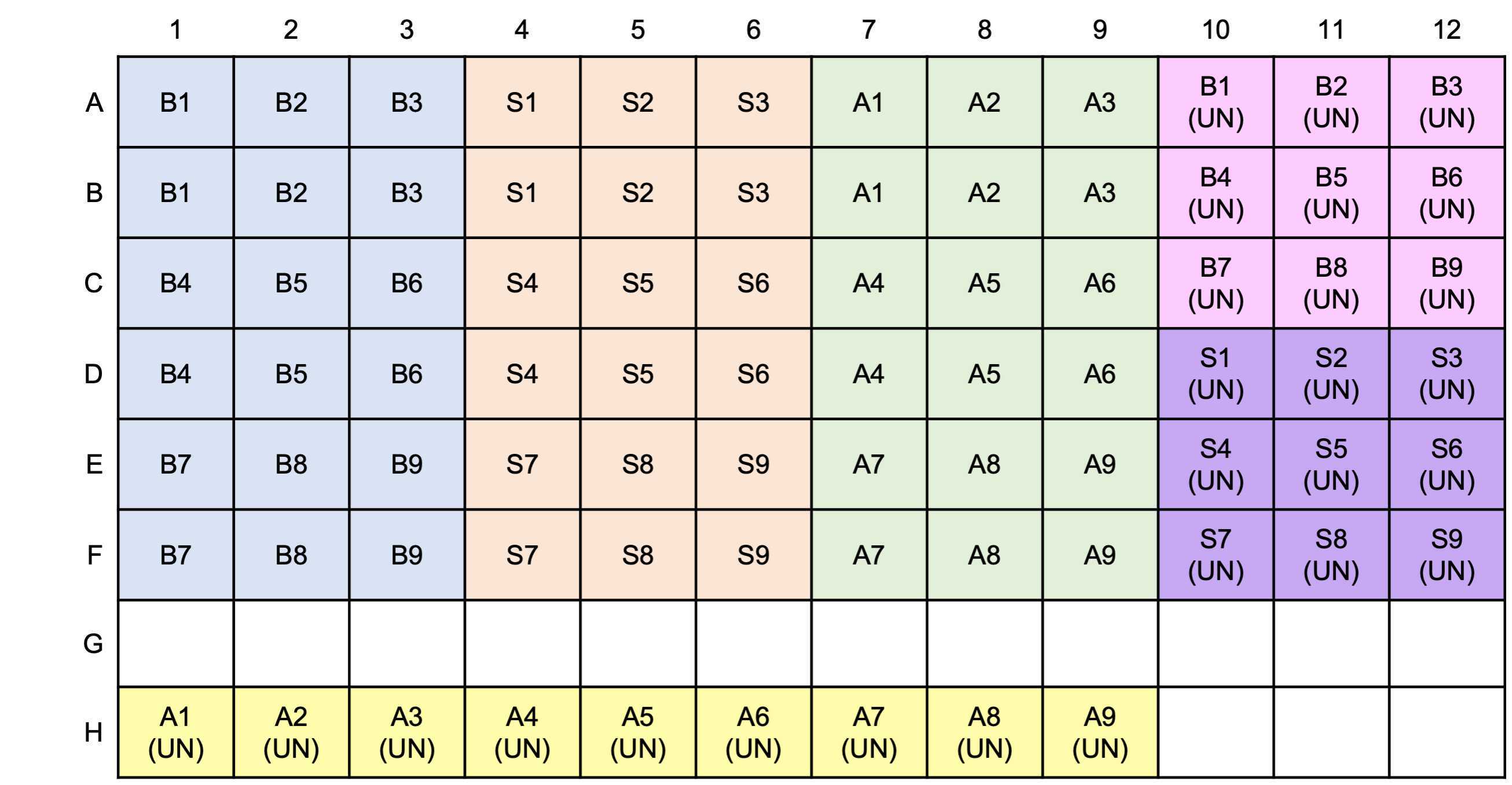
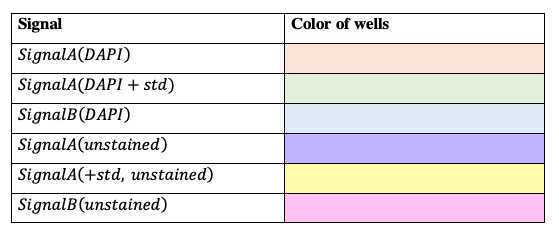
Load microtiter plate
Prepare DAPI working solution 100uM
Dilute 2.1µL of 14.3mM DAPI stock with 300µL MilliQ in a foil wrapped microtube and vortex.
Total volume = 30 X 54 (ul) for one microplate
12.6 ul 14.3mM DAPI stock with 1800µL MilliQ
Adhere black film on the top of a microplate lid and cover the plate with this lid.
Shake at room temperature.
Read fluorescence: excitation at 410 nm and emission at 550 nm