In situ high-speed brightfield imaging for studies of aquatic organisms
Sean P. Colin, Brad J. Gemmell, John H. Costello, Kelly R Sutherland
Abstract
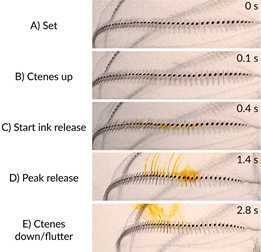
Behavioral measurements of fragile aquatic organisms require specialized in situ techniques.We developed an in situ brightfield camera set-up for use during SCUBA diving in aquatic ecosystems.The system uses brightfield illumination with collimated light and an underwater camera to highlight morphological details, body motion and interactions between organisms with high spatial (4K: 3840x2160 pixels) and temporal resolution (up to 120 fps).This technique is particularly useful for gelatinous organisms because of their large (centimeters in length), transparent bodies.Further, the measurements are not subject to experimental artifacts produced in laboratory studies.This method is useful for anyone seeking detailed brightfield images of organisms or nonliving material (e.g. marine snow) in the natural environment.
Before start
Before diving, test camera and housing in a sink or pool to ensure that it is leak proof.
Steps
Select field site
This system is lightweight, compact and portable and can be used SCUBA diving from shore, docks or boats.The camera system can also potentially be towed vertically from a research vessel.
Assemble equipment
The brightfield camera system relies entirely on available off the shelf components and does not require custom fabrication of components, which keeps the overall cost reasonable. The camera/lens and underwater housing configuration are flexible in that there are numerous configurations of commercially available platforms that will work in a brightfield setup allowing users to choose a frame rate and image resolution that best suits their needs. The illumination source must be far enough away from the light diffusing plate to ensure an even light distribution onto the plate or else uneven brightness will occur in the resultant images. A variety of light sources can be used but the user must consider that the optimal distance from the light to the diffuser will likely change between the light sources.

Image acquisition
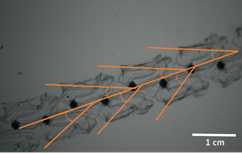
The subject of study (animal or interaction) can be centered in the field of view using the viewfinder.The video camera can be used successfully in fully automatic mode whereby shutter speed, the International Organization for Standardization (ISO), f-stop and focus are continually monitored and altered by the camera. Alternatively, any or all of the camera settings can be manually controlled to produce a better image, such as the focal plane, which is beneficial when filming low contrast, mobile targets. A photographic scale can be placed on the edge of the field of view helps focus the camera and also provides the spatial scaling information for subsequent measurements. A thin wire of known dimensions provides a useful scale without creating hydrodynamic disturbance. The illumination source has several intensity modes which can be changed underwater for on-the-fly adjustments to best match environmental conditions.

Post-processing
Video files can be viewed and converted to image stacks in Adobe Premiere or a similar video editing software.Image stacks can then be imported to ImageJ or a similar image analysis software for obtaining measurements from still images or image sequences.