Immunostaining and quantification of intracellular accessible cholesterol using ALOD4-mNeon in Human Fibroblasts
Suzanne R Pfeffer, Ebsy Jaimon, Sreeja V Nair
Abstract
Here we present a protocol for quantitative immunostaining of intracellular accessible cholesterol using ALOD4-mNeon in human fibroblasts.
Steps
ALOD4 Staining and image acquisition
This section describes plating and staining of cells and image acquisition.
Seed 0.2 X 106 cells onto 3-4 collagen-coated 12 mm coverslips, in each well of a six-well plate
16-18 hr after plating, wash the cells once with 1X PBS
Transfer each coverslip to a well of a 24-well plate containing 500µl 4% (v/v) PFA per well; fix the cells for 0h 15m 0s at Room temperature
Wash cells 3X with 1X PBS
Permeabilize the cells by addition of 0.1% Saponin in 1X PBS for 0h 5m 0s at Room temperature
Wash cells 3X with 1X PBS
Block fixative by addition of 1% BSA in 1X PBS for 0h 30m 0s at Room temperature
Dilute ALOD4-mNeon to 4µM in 1% BSA/1X PBS
Add 50 µl, 4µM ALOD4 per coverslip
Incubate cells for 1h 0m 0s at Room temperature
Wash cells 3X in 1X PBS and mount coverslips onto clean glass slides with 4 µl Mowiol. Air-dry coverslips overnight or at least 4-5 hours; store in dark at Room temperature
Images below were acquired using a Zeiss laser scanning microscope.
Batch process images for maximum intensity projection and background subtraction
Images need to be batch processed for maximum intensity projection and uniform background subtraction. Process the images for CellProfiler analysis as described in dx.doi.org/10.17504/protocols.io.3byl4bpo8vo5/v1
FOOTNOTE: For Zeiss images, the user needs to specify the folder to save the processed images and run lines 26, 27, and 29. Processing includes maximum intensity projection and background subtraction. Choose a value for background subtraction based on the images analyzed.
Import files and Segment Cells
In CellProfiler, select the Images module, drag and drop the background subtracted and maximum intensity projected, .TIF files created in Step 14 above. Here we subtracted a value of 50 to remove the background noise.
Select the Metadata module
In the Metadata module:
Extract metadata? Yes.
Metadata extraction method: Extract from image file headers
Extract metadata from: All images
Hit “Extract metadata”
Click Add another extraction method
Metadata extraction method: Extract from file/folder names
Metadata source: File name
Regular expression to extract from file name: “Regex” will be as follows: ^GM(?P[0-9]{4}) .*#(?P[0-9]{2}) for an example file name “GM01607.czi #01_max.tif”.
This step helps to extract the cell line, and position from each file name. In Regex, ^ indicates the beginning of the file name (?P[0-9]{4}) tells the program to name the captured field “Cell line” and recognize five digits that follow .* indicates any character (?P[0-9]{2}) tells the program to name the captured field “position” and recognize two digits that follow.
Metadata data type: Text
Hit “update” to populate the metadata field
Go to NamesAndTypes module
In the NamesAndTypes module:
Assign a name to: “Images matching rules”
Process as 3D: No
Select the rule criteria Match “All” of the following rules “Metadata/Does/Have C matching 0”
Name to assign these images: ALOD4
Select the image type: Grayscale image
Set intensity range from: Image metadata
Hit “update” to populate the names and types field
Go to Groups module
In the Groups module:
Do you want to group your images? Yes
Metadata category: Celltype
Metadata category: position
This groups images based on Cell type, and position as identified in the metadata module.
Segmentation of cells:
Click on the “+” sign at the bottom next to Adjust Modules. Under the module category, Image processing, Add RescaleIntensitymodule.
Select the input image: ALOD4
Name the output image: RescaleIntensity_ALOD4
Rescaling method: Divide each image by the same value
Divisor value: 0.001
Rescaling the intensity of images makes it easier to segment cells in the step below.
Add Identifyprimaryobjects module
In the Identifyprimaryobjects module:
Use advanced settings? Yes
Select the input image: RescaleIntensity_ALOD4
Name the primary objects to be identified: cells
Typical diameter of objects, in pixel units: 95 - 800
Discard objects outside the diameter range: Yes
Discard objects touching the border of the image: No
Threshold strategy: Global Thresholding method: Otsu
Two-class or three-class thresholding? Two classes
Threshold smoothing scale: 1.6
Threshold correction factor 1.0
Lower and upper bounds on threshold 0 and 1.0
Log transform before thresholding? Yes
Method to distinguish clumped objects? None
Fill holes in identified objects? After both thresholding and declumping
Handling of objects if excessive number of objects identified? Continue
Note: Check by clicking “Start Test Mode” and hitting the green triangle next to the IdentifyPrimaryObjects module each time a parameter is changed to find the best parameters for each image set.
Add OverlayOutlines module
In the OverlayOutlines module:
Display outlines on a blank image: No
Select image on which to display outlines: ALOD4
Name the output image: celloutline
Outline display mode: Color
How to outline: Thick
Select objects to display: cells
Select outline color: Maraschino
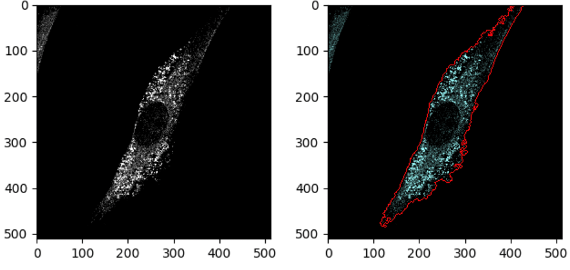
Measure ALOD4 intensity
Add MeasureObjectIntensity module
In the MeasureObjectIntensity module:
Select images to measure: ALOD4
Select objects to measure: cells
This module measures ALOD4 intensity in segmented cells.
Add MeasureObjectSizeShape module
In the MeasureObjectSizeShape module:
Select object sets to measure: cells
Calculate Zernike feature? No
Calculate the advanced features? No
Add ExportToSpreadsheet module from the + at the bottom
Select the column delimiter: tab
Output file location: choose a folder where you want the images to be saved
Add a prefix to file names? Yes
File name prefix: Add experiment identifier
Overwrite existing files without warning? No
Note: While the pipeline is run for optimizing the parameters, choose Yes to avoid being asked to rewrite each file.
Add image metadata columns to your object data file? Yes
Add image file and folder names to your object data file? Yes
Representation of Nan/Inf: NaN
Select measurements to export? Yes
Press button to select measurements: Under “cells” choose AreaShape -> Area, and Intensity -> Integrated Intensity.
Calculate the per-image mean values for object measurements? No
Calculate the per-image median values for object measurements? No
Calculate the per-image standard deviation values for object measurements? No
Create GenePattern GCT file? No Export all measurement types? No
Data to export: cells Use the object name for the file name? Yes
Save the pipeline from File-Save Project and hit Analyze Images on bottom left.
The pipeline will run and export the data to the folder previously specified. The output file can be opened using Excel software. Columns indicate Cell line, image number, area of each cell, and integrated intensity of ALOD4 in each cell.
#尊敬的用户,由于网络监管政策的限制,部分内容暂时无法在本网站直接浏览。我们已经为您准备了相关原始数据和链接,感谢您的理解与支持。
https://lh7-us.googleusercontent.com/docsz/AD_4nXeb0ZBTqCz9XPUddqfJ_jLv7Uce2hjNakr4SOONgX0glsLfEju56rYQCNW_sRrEhVFDsQlXYmaF-WW45Zv1beKsJ94k6qFdPzFNlNVd4dKdoWx6O6ckRkNCmKbSsDseiAJENZxXl78ibn9C5McpsAAWOpNj?key=rXYczieUNaoKcQ2q5v_nDg

