Immunocytochemistry for the characterization of hiPSC to Motor Neuron differentiation
William J Buchser, Mallory Wright, Colin Kremitzki
hiPSC (Human induced pluripotent stem cells)
Immunocytochemistry
Immunofluorescence
Neural differentiation
Motor neuron differentiation
Abstract
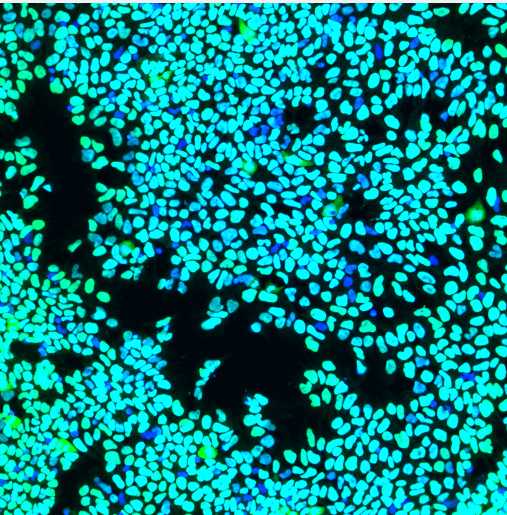
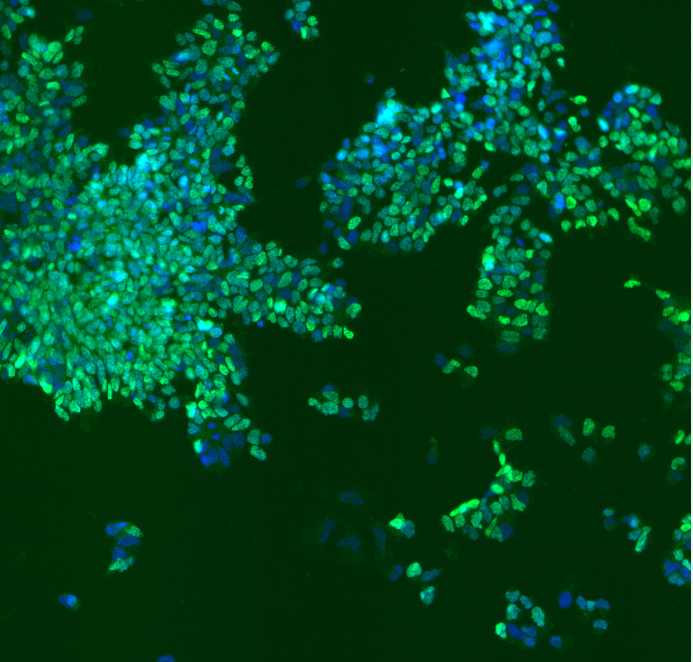
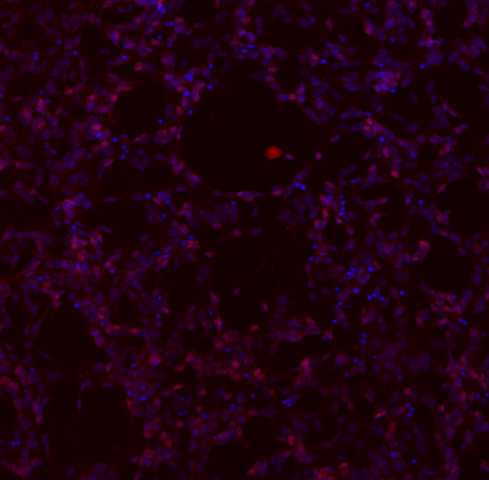
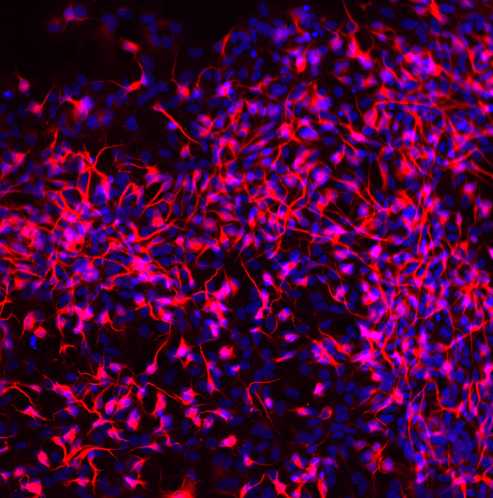
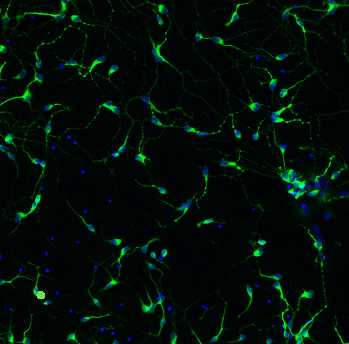
This immunocytochemistry protocol is used for the characterization of IPSC differentiation into motor neurons using several biomarkers: neuroepithelial cells (SOX1), motor neuron progenitors (OLIG2 and NKX2.2), motor neurons (MNX1), and the mature motor neurons (ISL, ChAT, MAP2).
*Primary and secondary antibody information located in materials section

Before start
To ensure that the observed results are not just random events, use controls, such as undifferentiated IPSCs when doing immunocytochemistry.
Here is an example of how we seed cells onto a 96-well plate. The plate has an equal amount of wells of differentiated (blue) and undifferentiated cells (yellow) for analysis, and we always include a moat (evaporation barrier). The cells are also counted and seeded at equal densities.
Steps
Remove the medium from your cells
FIX - Dilute 32% Paraformaldehyde solution to 4% PFA in 1X phosphate-buffered saline (PBS)
Add 100uL of 4% PFA to each well in the 96-well plate. (1ml if using a 6-well plate). Incubate for 0h 15m 0s at room temperature.
Remove the fixative solution and wash with 1XPBS at 100ul per well using a multichannel pipette. Repeat 3 times.
PERMEABILIZE - Add 100uL of 0.5% Triton X-100 to each well of a 96-well (1ml if using 6-well plate)
Incubate for 0h 15m 0s at room temperature.
Remove the permeabilization solution and wash 3 times with 1XPBS
BLOCK - Add 100ul of 3% BSA (bovine serum albumin) or blockAid-blocking solution to each well of a 96-well plate slowly to Block. (1ml if using 6-well plate)
Incubate for at least1h 0m 0s (up to overnight) at room temperature.
Calculate the amount of primary antibody needed (located in materials section) and dilute in 3% BSA + 0.3% Triton X-100 solution
PRIMARY ANTIBODIES - Remove 3% BSA from wells and add 100uL of primary antibody per well
Incubate for 1h 0m 0s at room temperature in a dark place or overnight at 4°C.
(Different primary antibody stains may take longer to stain and could require optimization).
Remove primary antibody and wash three times slowly with 1xPBS
SECONDARY ANTIBODIES - Calculate amount of secondary antibody needed (located in materials section) and dilute in 3% BSA+ .3% TritonX-100 solution and 1:4000 Hoechst.
Add 100uL per well in 96-well and incubate for at least 1h 0m 0s at room temperature in a dark place.
Remove secondary antibody and wash three times with 1xPBS
Scan on the confocal microscope