Human ovarian tissue fixation
hannah.anvari, francesca.e.duncan duncan, Pooja Devrukhkar
Abstract
Purpose: This protocol is intended for use in fixing human ovarian tissue in a research setting. This protocol supplements the steps detailed in the collection, processing, and long-term storage of human ovarian tissue as formalin-fixed paraffin-embedded (FFPE) samples in protocol: dx.doi.org/10.17504/protocols.io.e6nvwdk29lmk/v1.
Steps
Ovarian tissue fixation for fixed wedges and ovarian explant pieces
Fill 1.5 mL micro centrifuge tube with 1 mL of Modified Davidson’s Solution (Cat. #64133- 50; Electron Microscopy Sciences, Hatfield, PA)
Add one ovary wedge or all tissue pieces (ovarian explant) from one experimental well per 1.5 mL micro centrifuge tube.
Allow all tubes to rock @ room temperature (RT) for 2-4 hrs.
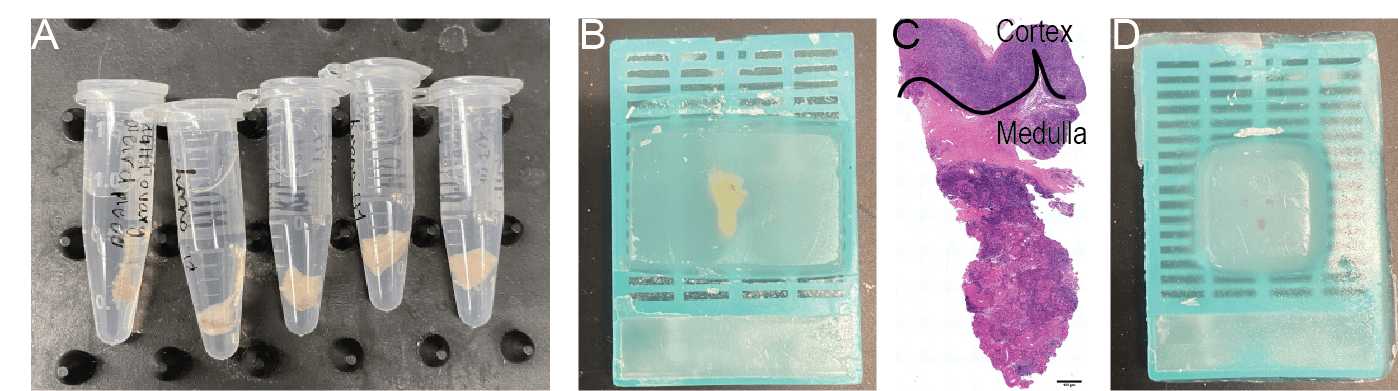
Transfer tubes to cold room and rock at 4°C overnight (Fig. 1).
Wash in 1 mL 70% EtOH 3x10 minutes, rocking in between each, and then store in 70% EtOH @ 4°C until processing/paraffin embedding, no longer than 1 week.
Ovarian tissue wedges containing both cortex and medulla should be embedded so that the cortex and medulla will be sectioned in one plane (Fig. 1B). A representative H&E tissue section indicates the proper orientation (Fig. 1C).
Ovarian tissue pieces from explant culture should be embedded together, if cultured in the same well (Fig. 1D). While embedding try to ensure that tissues sit as evenly as possible, so that they are sectioned in the same plane.

