HuBMAP | VU TMC Eye/Pancreas | Human Pancreas Processing for Multiple Applications
Carrie Romer, Jamie Allen, Jeff Spraggins, Danielle Gutierrez, Diane Saunders, Angela R.S. Kruse, Rita Bottino, Marcela Brissova, Alvin C. Powers
HuBMAP
Vanderbilt
Pancreas
Paraffin-embedding
cryosections
Flash frozen sections
RNA isolation
BIOMIC
Disclaimer
Developed in collaboration with Dr. Rita Bottino (Imagine Pharma) and Dr. Marcela Brissova (Vanderbilt University); adapted from this standard operating procedure established by the Network for Pancreatic Organ Donors with Diabetes (nPOD).
Abstract
This protocol describes the workflow for collecting human pancreas samples for multimodal downstream assays, in use by the Vanderbilt University HuBMAP Tissue Mapping Center.
Steps
Prepare reagents, tubes, labels, etc. as outlined in the template linked below.
Prepare solutions as follows.
-
100 mM PBS, 1L: Add 12.07 g Na2HPO4 (dibasic), 2.04 g KH2PO4 (monobasic), 8.0 g NaCl, and 2.0 g KCl to 1L glass bottle, then add MilliQ water to volume. Place on stir plate until dissolved. Filter through a 0.22 μm filter and store at 4ºC for up to 1 month.
-
70% Ethanol, 1L: Combine 700 mL of 190 Proof ethanol with 300 mL of dH2O.
-
16% Paraformaldehyde (PFA), for FCMC: Add 60 mL of 16% PFA stock into a 500 mL glass bottle, then add 180 mL of 100 mM PBS. Mix gently and transfer 40-mL aliquots into prelabeled 50-mL conical tubes for fixation. ⚠ This solution should be made fresh within 12 hours of use.
-
16% Paraformaldehyde (PFA), for CLR: Add 20 mL of freshly-opened 32% PFA ampule into a 500 mL glass bottle, then add 140 mL of 100 mM PBS. Mix gently and transfer 30-mL aliquots into prelabeled 50-mL conical tubes for fixation. ⚠ This solution must be made fresh immediately prior to use.
-
30% Sucrose: Add 72 g sucrose to approximately 100 mL of 10 mM PBS in a 500 mL glass bottle. Place on stir plate until dissolved, then bring to 240 mL total volume with 10 mM PBS. Filter through a 0.22 μm filter and store at 4ºC for up to 1 month.
-
2.6% Carboxymethylcellulose (CMC) : Add 500 mL water to 500 mL glass bottle and microwave for 2 minutes. Place bottle on heated stir plate (~70ºC), and slowly add 13g CMC to warmed water. Stir on low hotplate overnight, occasionally tightening lid and shaking bottle. Store at 4ºC for up to 2 months. Before each use, pour solution into 125 mL bottle and sonicate for 10 minutes.
-
10% Neutral Buffered Formalin: Pipet 18-mL aliquots into prelabeled 50-mL conical tubes.
-
RNAlater™ Stabilization Solution: later ™ Stabilization Solution: Pipet 40-mL aliquots into prelabeled 50-mL conical tubes.
Organ Dissection and Cross-Sectioning
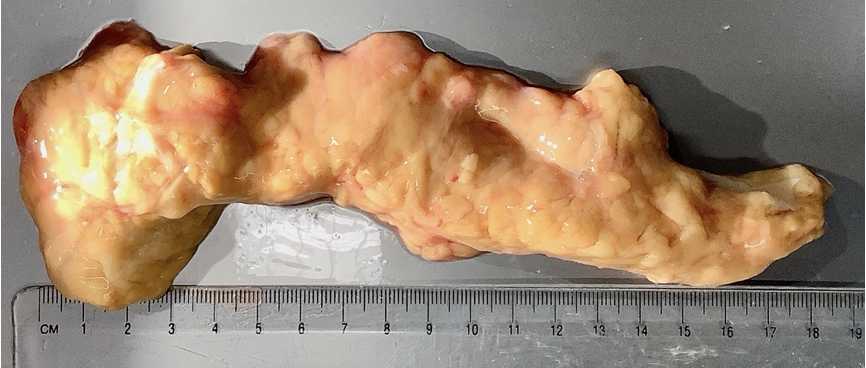
Upon arrival, remove the pancreas from transport solution (UW or HTK) and transfer into a large dissection tray placed on ice. Photograph the organ and note the following prior to dissection:
Gross morphology:
- Peri-pancreatic fat: normal or excessive?
- Peri-pancreatic adhesions : yes or no?
- Texture: soft, firm, or hard? Any palpable masses?
- Outer surface: is contour smooth or irregular? Note any localized bulge, hemorrhage, breakdown, chalk-white areas, or changes in color.
- Other external features: describe as needed.
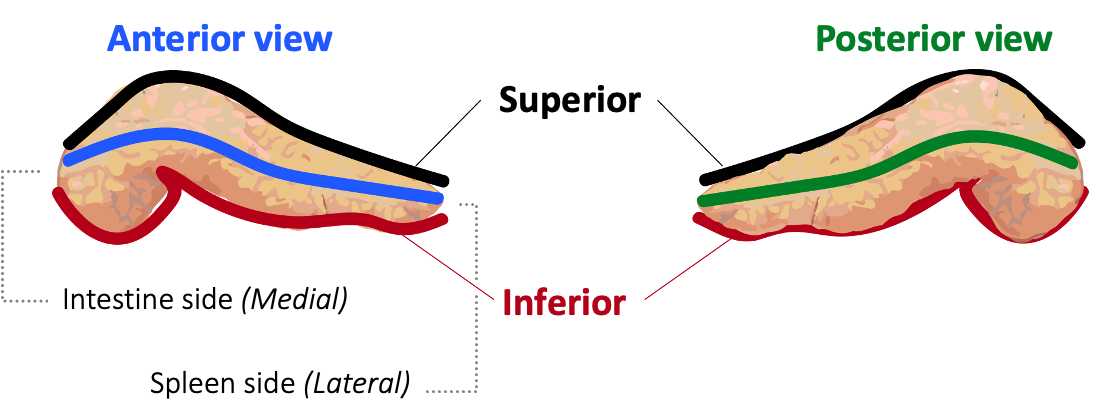
Dissect the pancreas by carefully removing duodenum, mesentery, fat, and spleen ( Figure 1 ).
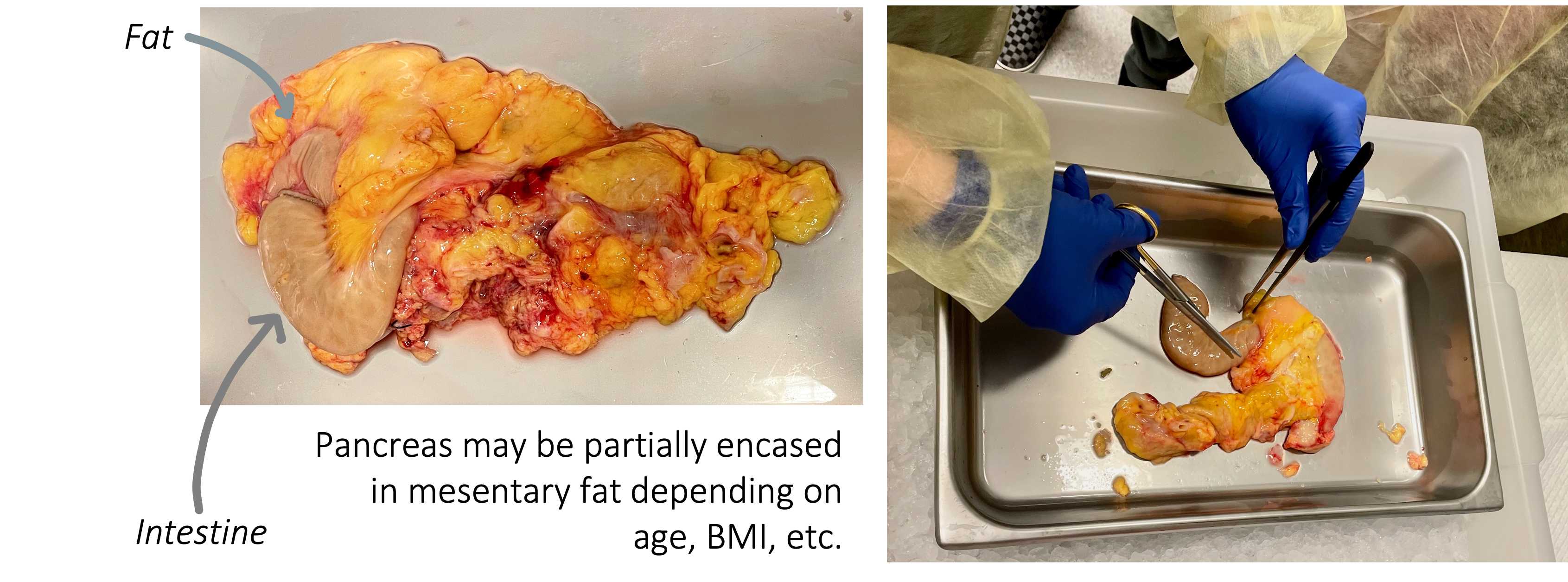
⚠ Note: If time and personnel permit and there are pieces of duodenum and/or spleen, retain and freeze as tissue blocks (see Flash Freezing subheading below).
Once the pancreas is cleaned, blot with a sterile gauze square and quickly transfer to a clean plastic weigh boat. Place on a pre-tared balance and record pancreas weight .
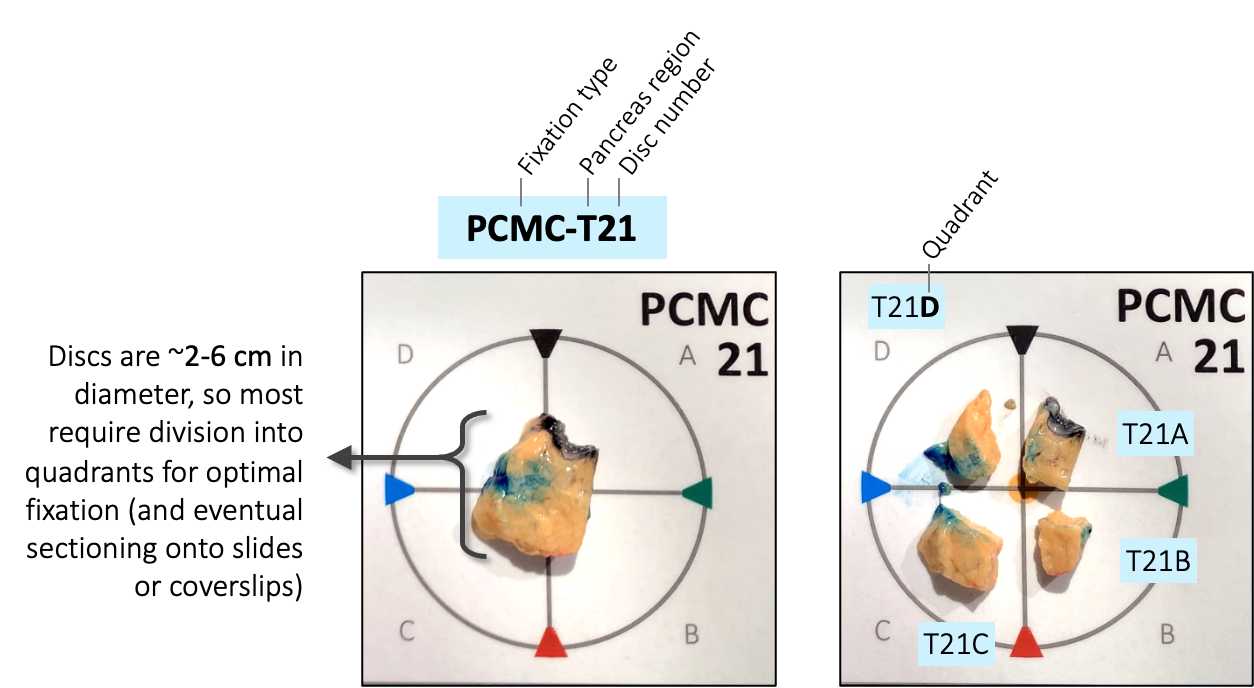
Once the pancreas has been marked, start collecting approximately 5 mm cross-sectional slices ("discs") starting from the head (duodenum) and moving laterally towards the tail (spleen) as outlined in Figure 4 . Each disc should contain dye markings on the exterior to provide spatial orientation.
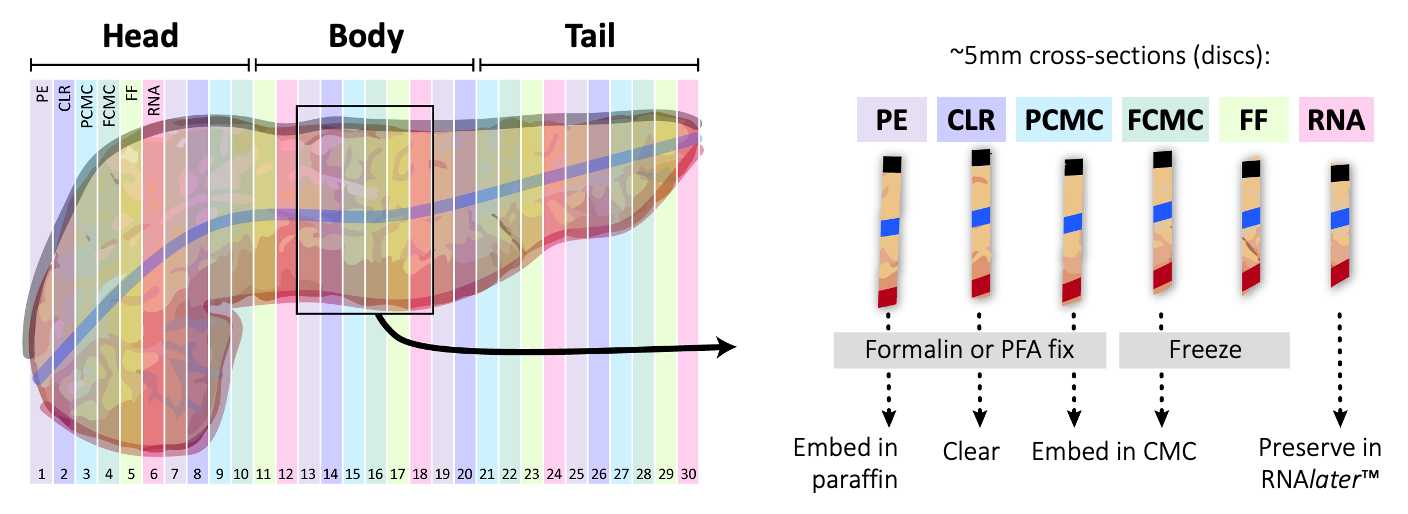
Photograph each disc, medial side down, using prelabeled cards with nomenclature developed for Vanderbilt LIMS (encodes positional information and processing type).
As discs are photoraphed, make notes on the experimental spreadsheet if any of the following gross morphology is observed:
- Dilated ducts, cystic change, or stones
- Hemorrhage
- Tissue breakdown (necrosis)
- Chalk-white areas (calcification)
- Visible neoplasms
- Other features
Divide each disc into four approximately equal-sized quadrants, attempting to bisect dye markings so that each tissue piece contains 2 dye colors ( Figure 5 ). Photograph disc again after quadrants are cut.
Transfer each set of quadrants to the appropriate processing protocol as outlined in Figure 4 .
Formalin Fixation and Paraffin Embedding (PE)
Place all tissue quadrants from one disc into a 50-mL Falcon tube containing 40mL of
Fix or at least 18h 0m 0s at 4°C under mild agitation using an adjustable tilt rocker (LabNet).
Wash tissue four times in 40mL of 100 mM PBS at 4°C for a period of 3h 0m 0s, rocking. Blot the tube with paper towel before adding the fresh washing solution.
After the last wash, add 40mL of 70% ethanol and store at 4°C.
To process for paraffin embedding, transfer fixed tissue samples from 50-mL tube into a 10-cm petri dish. Using the photographs described in step #10 and tissue marking dyes, identify quadrants A-D. Place each quadrant, medial side down, into prelabeled
Follow schedule as follows using Epredia™ Excelsior™ AS Tissue Processor (total 16 hours):
- 70% Ethanol, one change -
0h 45m 0s - 90% Ethanol, one change -
0h 45m 0s - 95% Ethanol, one change -
0h 45m 0s - 100% Ethanol, three changes -
0h 35m 0seach - Xylene or xylene substitute (e.g., Clear Rite 3), three changes -
0h 35m 0seach - Paraffin wax,
58°Cto60°C, three changes -1h 0m 0seach
Trim blocks as necessary and store at Room temperature.
Light PFA Fixation and CMC Embedding (PCMC)
Place all tissue quadrants from one disc into a 50-mL Falcon tube containing 40mL of freshly prepared fixative (4% PFA*/100 mM PBS).
*
Fix for 3h 0m 0s On ice under mild agitation using an adjustable tilt rocker (LabNet).
Wash tissue four times in 40mL of 100 mM PBS On ice for a period of 2-3 hours (0h 30m 0s per wash), rocking. Blot the tube with paper towel before adding the fresh washing solution.
After the last wash, equilibrate the tissue in 40mL of sucrose solution (30% sucrose/10 mM PBS) at 4°C 0h 45m 0s. Tissue will settle to bottom of the tube.
Fill a prelabeled
Pick each quadrant up with a pair of fine forceps, blot on a Kimwipe to remove excess sucrose, and place into the appropriate CMC-prepared cryomold. Using the forceps, push the tissue lightly to bottom of the cryomold, medial side down.
Transfer the cryomold to a dry ice block to freeze, adding more CMC to completely fill the indentation. When CMC compound is completely frozen, wrap each tissue block tightly in a pre-labeled aluminum foil and place it a storage bag to prevent it from drying out.
Store samples at -80°C.
▷ If sample transfer is required, ship blocks on dry ice.
Flash Freezing +/- CMC Embedding (FF, FCMC)
Prepare isopentane slurry:
Place a layer of dry ice into an insulated, lidded container and add enough
Place each tissue quadrant into a prelabeled
For FF samples (no embedding), wrap frozen tissue samples tightly in prelabeled squares of aluminum foil.
For FCMC , apply a thin layer of CMC into prelabeled
Place all wrapped samples in prelabeled storage bags and store at -80°C.
▷ If sample transfer is required, ship blocks on dry ice.
Preservation for Spatial Transcriptomics (RNA)
Place all tissue quadrants from one disc into a 50-mL Falcon tube with approximately 5 volumes of
18mL).
Store samples at 4°C.
▷ If sample transfer is required, ship sealed tubes on ice packs.
PFA Fixation for Tissue Clearing (CLR)
Place all tissue quadrants from one disc into a 50-mL Falcon tube containing 40mL of freshly prepared fixative (4% PFA*/0.1 M PBS).
*
Fix for 16h 0m 0s at 4°C under mild agitation using an adjustable tilt rocker (LabNet).
Wash tissue three times in 40mL of 100 mM PBS 4On ice under mild agitation.
Continue immediately to tissue clearing protocol, or store samples at 4°C in 100 mM PBS containing 0.1% sodium azide.