Hematoxylin and Eosin (H&E) Staining of Tissues following Multiplexed Imaging on Phenocycler-Fusion
William F Flynn, Elise T Courtois, Santhosh Sivajothi, Emily Soja
Abstract
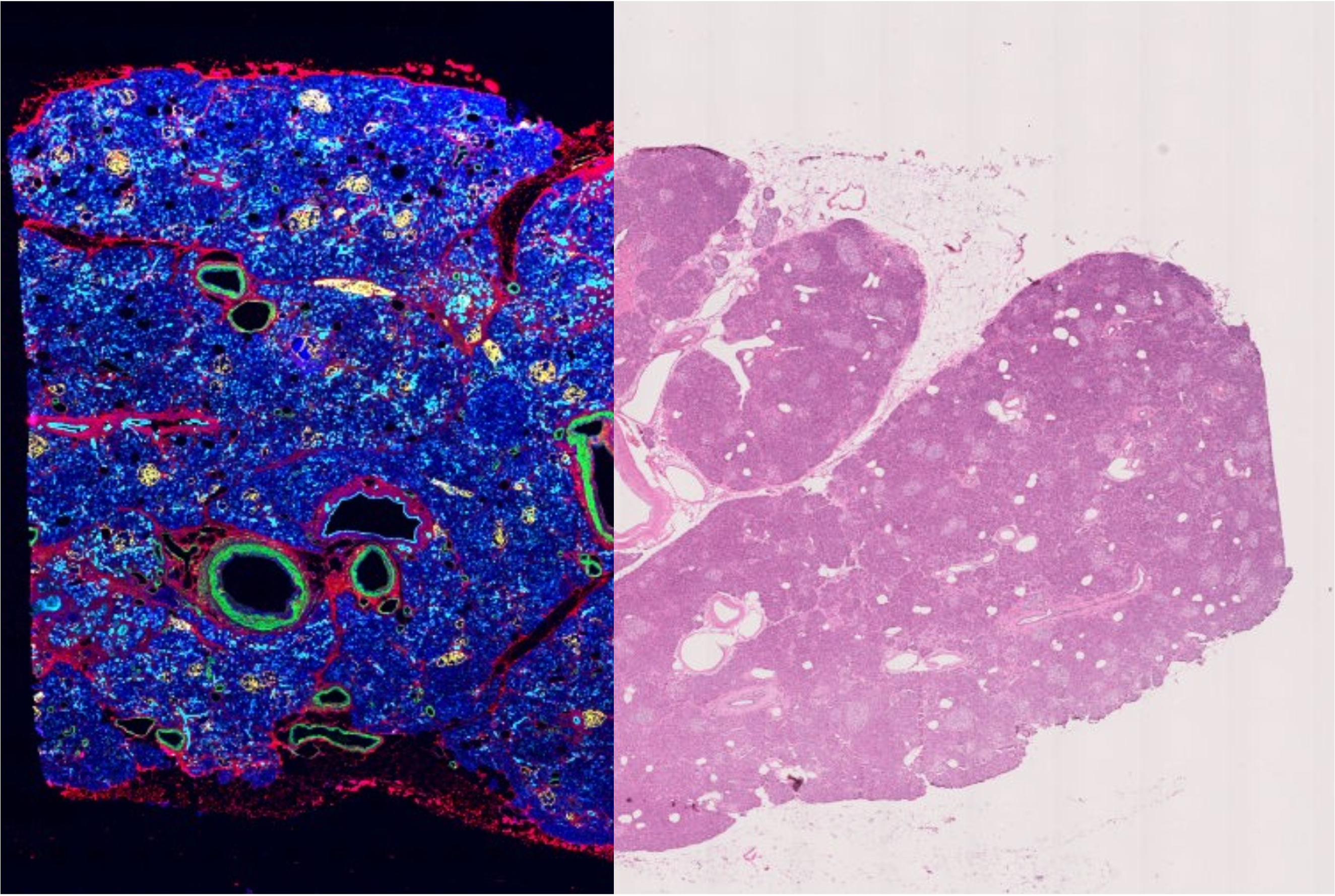
Phenocycler-Fusion assay enables multiplexed antibody based imaging of FFPE or fresh frozen tissues. Hematoxylin and Eosin (H&E) staining is a gold standard method for observing tissue morphology. Obtaining multiplexed protein expression information and H&E images from the same tissue provides an additional layer of valuable information which can be used to interpret imaging data and used as input novel machine learning algorithms. This protocol details the steps to generate high quality H&E images from both FFPE and fresh frozen (FF) tissues following multiplexed imaging on the Phenocycler-Fusion.
This protocol can also be used for H&E staining following multiplexed imaging on other platforms. In that case, skip the 'Flow cell removal' section, perform two washes of 0h 5m 0s each in 1X PBS and proceed directly to Step 5. Step 5 .
Steps
Flow cell removal
After a successful Phenocycler run, slides with attached flow cells can be stored in the storage buffer at 4°C or used immediately for H&E staining.
The flow cell needs to be removed from the slide in order to continue with H&E staining. Retrieve tissue slides with attached flow cells either from the storage buffer or directly after Phenocycler run.
Fill a coplin jar with xylene or Histo-Clear. There must be sufficient volume to cover the entire flow cell.
Place slides in the coplin jar containing xylene or Histo-Clear and incubate for 24h 0m 0s at Room temperature
Following incubation, gently remove the flow cell from the slide.
H & E staining
Place the slides in 100% ethanol for 0h 2m 0s. Raise and dip the slides 10 to 15 times to fully cover the tissue.
Place the slides in 95% ethanol for 0h 2m 0s. Raise and dip the slides 10 to 15 times to fully cover the tissue.
Place the slides in DI water for 0h 2m 0s. Raise and dip the slides 10 to 15 times to fully cover the tissue.
Proceed immediately from step 5 and place the slides in the following reagents:
Mayer’s Hematoxylin - 0h 4m 0s
Xylene/Histo-Clear - Hold in this solution as slides are mounted one by one
DI water - 0h 1m 0s
Bluing Reagent - 0h 1m 0s
DI water - 0h 1m 0s
Alcoholic Eosin – 0h 2m 0s
95% ethanol - 0h 1m 0s without agitation or 20-30 dips to remove all excess reagent
from previous step
100% ethanol - 0h 1m 0s without agitation or 20-30 dips to remove all excess reagent from previous step
100% ethanol - 0h 1m 0s without agitation or 20-30 dips to remove all excess reagent from previous step
Xylene/Histo-Clear - 0h 1m 0s without agitation or 20-30 dips to remove all excess reagent from previous step
Slide mounting
To mount the slides, remove slides one at a time on to a paper towel inside the fume hood. Leave the other slides in the last xylene/Histo-Clear coplin jar to prevent over-drying the tissue.
Dry the back of the slide and tilt to remove excess xylene, but do not let the tissues dry.
Clean a glass coverslip quickly and remove any particles on its surface. Place the long edge of the coverslip on the edge of the slide closest to you, tip the slide towards yourself so the mountant begins to reach the coverslip, and gently push down until the mounting media has spread evenly across the slide area containing tissue.
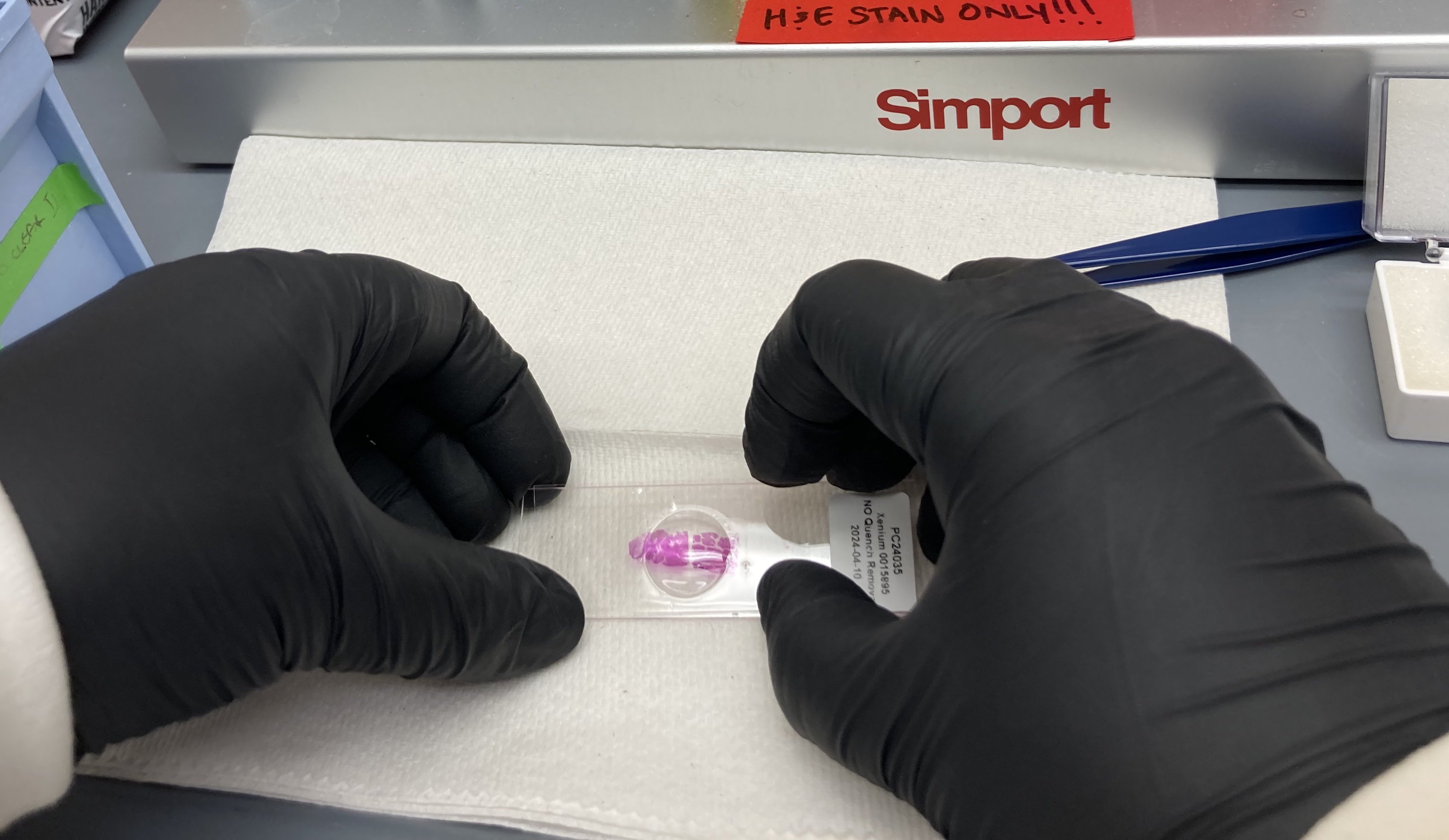
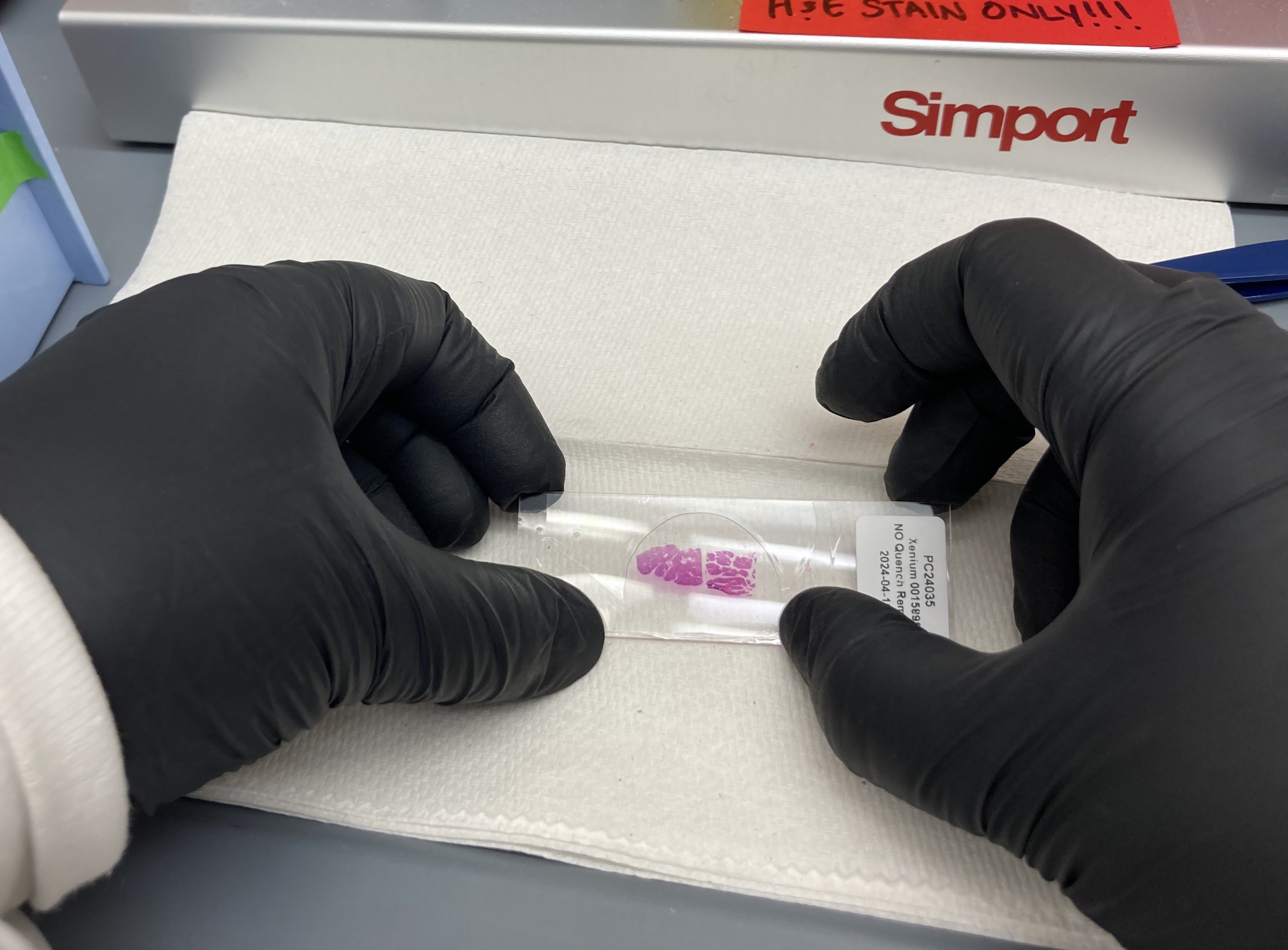
Gently press down on the coverslip to remove any bubbles that remain above the stained tissue.
Allow mountant to cure inside the hood for at least 0h 20m 0s before moving and imaging. Xylene based mountants take up to 24 hours to cure completely.
and repeat mounting procedure for remaining slides.
Mounted slides can be imaged and stored in a slide storage box at Room temperature
Expected Result:
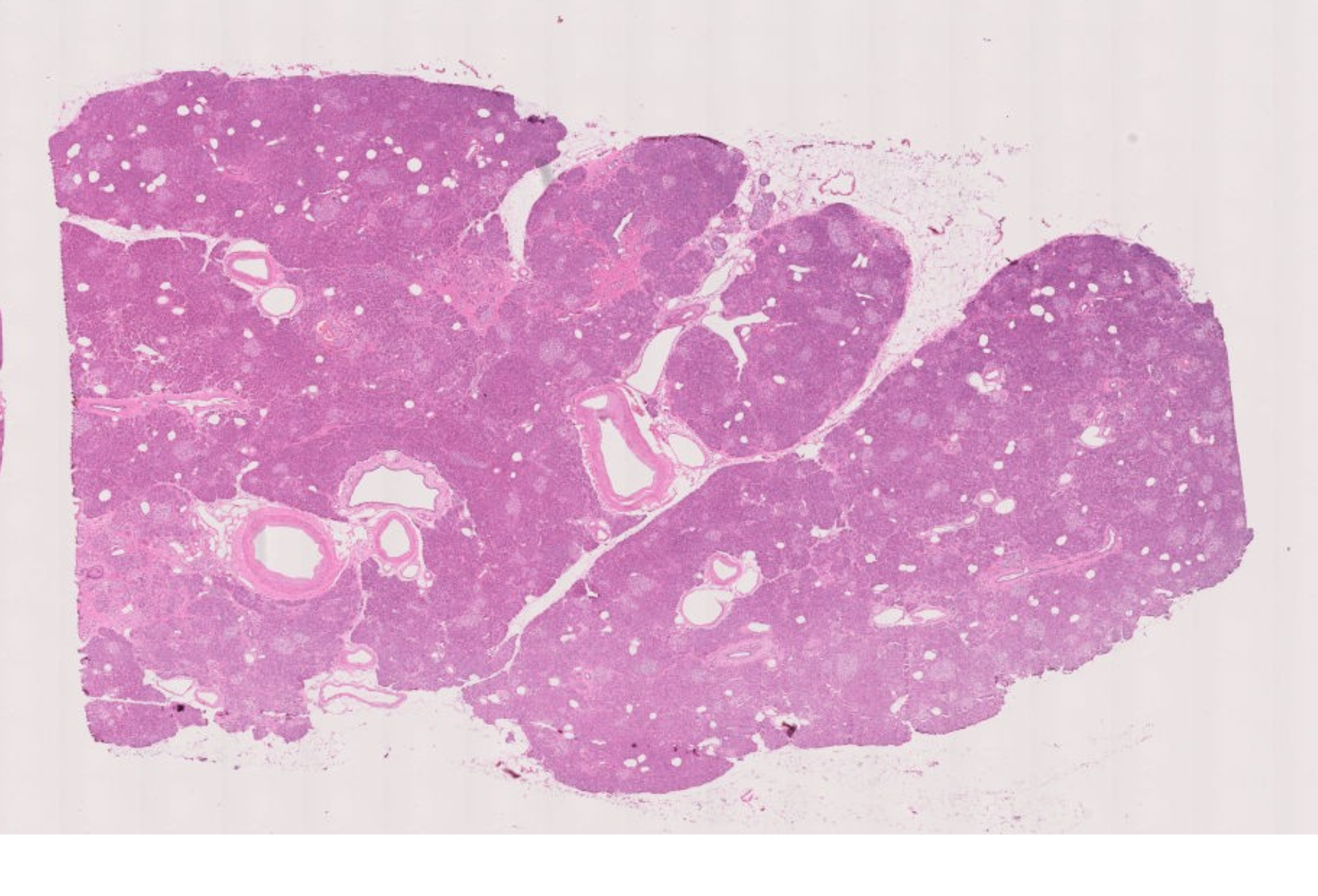
H&E stained image of human pancreas FFPE tissue following multiplexed imaging on Phenocycler-Fusion ( Data courtesy of Prof. Paul Robson, The Jackson Laboratory )