Freshwater mussel eDNA: water sampling and filtration through Sterivex filter unit
Marine Vautier
Abstract
The objective of this protocol is the sampling and the filtration of water samples through 0.45 µm Sterivex™ filter units . This protocol is used upstream to molecular biology analysis (e.g. qPCR, metabarcoding, ddPCR) to specifically target freshwater mussel eDNA .
Once filtered, Sterivex filter units can be stored at room temperature or at 4°C for periods ranging from a few days to several months depending on the preservation buffer used (e.g. Longmire, CTAB, C1) or can be frozen directly without the addition of a preservation buffer for long preservation .
This protocol allows to filter approximately between 0.5 L and 5 L through a Sterivex™ filter unit depending on the water sample characteristics and the chosen filtration method (syringe or vacuum pump).
Before start
- Prior to sampling, the bottles and filtration material dedicated to eDNA have to be decontaminated . The following protocol can be applied:
Hydrogen peroxide decontamination of eDNA dedicated material
-
Store all materials in clean bags or containers to reduce the risk of contamination.
-
Wear gloves during the whole procedure
-
During the handling try to reduce exposure to sunlight if possible.
Steps
WATER SAMPLING
Wear gloves and open the bottle without touching the inside of the cap or the neck of the bottle.
- Several options are available:
-
Surface sampling :Collect by hand sub-surface water (10-20 cm below the water surface) and close the bottle carefully.
-
Depth sampling : If water is collected deeper using a sampling container (e.g. Niskin bottle), it must also be decontaminated before sampling. Transfer the contents of the tank into the bottle to minimise the risk of contamination.
-
After sampling, store the bottle in a clean container to avoid contamination.
-
Use a cooler if samples need to be transported.
WATER FILTRATION
Proceed rapidly to water filtration in order to minimise any potential DNA damage during transport. Place the water at 4°C in the dark for max. 6 hours until filtration.
- There are two filtration protocols , one manual using a syringe , and the other using a vacuum pump . Both protocols are described below:
Filtration procedure with a syringe :
Set up is simple and does not necessarily require bench space (can be done in the field). Prepare the filtration material: gloves, Sterivex cartridge, caps and syringe.
-
Put on gloves and remove the syringe and Sterivex from the blister pack (handle carefully, avoiding touching either end of the filter to avoid contamination).
-
Gently homogenise the water sample & fill the syringe (60ml) with the water sample (check that the neck of the bottle is larger than the diameter of the syringe).
-
Connect the syringe to the Sterivex (Figure 1)
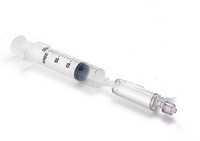

-
Filter (slowly) the water sample through the Sterivex unit.
-
Remove the syringe from the Sterivex to refill the syringe with water and filter through the same Sterivex.
-
Continue the filtration (using the same syringe and Sterivex) to filter a maximum volume of water and note the final volume of water filtered (e.g. by subtracting the remaining volume from the initial volume of water). Typical filtered volumes range from 0.5L to 5L: the volume of water filtered will depend on the microbial load and turbidity of the water sample.
Note: It is recommended that filtration does not exceed 30 minutes to limit the risk of degradation of the DNA sample on the filter.
-
At the end of filtration, remove the Sterivex from the syringe and remove as much of the residual water as possible by inserting the syringe plunger and passing air through the Sterivex.
-
Proceed to sample preservation (Step 3)
Filtration procedure with a vacuum pump :
The set-up does not necessarily require bench space, but is easier to set up in a laboratory. Prepare the filtration material: vacuum pump, tubing, filter ramp adapted to the size of the Sterivex, gloves, Sterivex cartridge, caps and syringe.
-
Wear gloves and remove the Sterivex from the blister pack (handle carefully, avoiding touching either end of the filter to avoid contamination).
-
Insert the male Luer lock outlet tip of the Sterivex (Figure 2) into the clean silicone filter holder (Figure 3).

-
Place the clean silicone filter holder onto the filtration ramp (Figure 3).
-
Remove the 50ml syringe from the blister pack and remove the plunger from the syringe to use the syringe body as a funnel (leave the plunger in the blister pack to use at the end of the filtration process).
-
Connect the body of the syringe to the female Luer lock inlet tip of the Sterivex (Figure 2 & 3).
-
Gently homogenise the water sample.
-
Slowly fill the syringe with water directly from the bottle as if it were a funnel (Figure 3).
-
Switch on the pump and open the valves (the pressure must not exceed 500 mbar (50 kPa)).
-
Fill the syringe body (funnel) to ensure that there is always water in it.
-
Continue the filtration (using the same syringe and Sterivex) to filter a maximum volume of water and note the final volume of water filtered. Typical filtered volumes range from 0.5L to 5L: the volume of water filtered will depend on the microbial load and turbidity of the water sample.
Note: It is recommended that filtration does not exceed 30 minutes to limit the risk of degradation of the DNA sample on the filter.
-
At the end of filtration, remove the Sterivex from the silicone filter holder.
-
Remove as much of the residual water as possible by inserting the syringe plunger and passing air through the Sterivex.
-
Proceed to sample preservation (step 3)
Preservation of the sample
There are two possible preservation methods , One by using a preservation buffer , the other by freezing the Sterivex directly . Both protocols are described below:
Preservation with a preservation buffer
Prepare the preservation material: buffer (e.g. Longmire, CTAB, C1), Sterivex caps, 1000µL pipette or 5 mL seringe, indelible marker or pre-printed label, sterile bag (or tube) to store the Sterivex after filtration, field sheet and pen.
-
Place the outlet cap on the Sterivex (Figure 2).
-
Add immediately 2mL of buffer using a pipette (tip 1000µL) or a 5 mL seringue. Be careful to well insert the tip into the Sterivex to ensure the buffer does not overflow the cartridge (Figure 4). Caution : it is important not to put more than 2 mL of buffer into the Sterivex because reagents will be added during the DNA extraction step.
Note : Change the tip between pipetting operations to avoid contaminating the tube with preservative buffer.

-
Place the Inlet cap on the Sterivex (Figure 2).
-
Place the labelled Sterivex in a clean bag/tube.
-
Sterivex buffered can be stored at room temperature or at 4°C for periods ranging from a few days to several months depending on the buffer used (e.g. Longmire, CTAB, C1).
-
Complete the field sheet.
Preservation without preservation buffer
Prepare the material: Sterivex caps, indelible marker or pre-printed label, sterile bag (or tube) to store the Sterivex after filtration, field sheet and pen.
-
Place the outlet and the inlet caps on the Sterivex (Figure 2).
-
Place the labelled Sterivex in a clean bag/tube.
-
Freeze the Sterivex immediately after filtration. This can be done either by placing it immediately in a freezer (-20°C or -80°C) or in liquid nitrogen.
-
Complete the field sheet.

