Flow cytometry trait measurements (size, granularity and chlorophyll-a) of diatoms
Phoebe Argyle, Jana Hinners, Naomi M. Levine, Sinead Collins, Nathan Walworth, Martina Doblin
Disclaimer
Abstract
A method and guide for measuring size, relative granularity, and chlorophyll-a content in centric diatom cells. This method was developed on a CytoFlex LX instrument with Thalassiosira spp. as the model organism.
This protocol was used in the following publications:
Argyle, P. A., Walworth, N. G., Hinners, J., Collins, S., Levine, N. M., & Doblin, M. A. (2021). Multivariate trait analysis reveals diatom plasticity constrained to a reduced set of biological axes. ISME Communications , 1 (1), 59.
Argyle, P. A., Hinners, J., Walworth, N. G., Collins, S., Levine, N. M., & Doblin, M. A. (2021). A high-throughput assay for quantifying phenotypic traits of microalgae. Frontiers in microbiology , 12 , 706235.
Steps
Sample preparation
Aliquot 200µL of live, uniform culture and transfer to a round-bottom tissue culture plate. Agitate culture gently to suspend cells to create a uniform culture.
Equipment
| Value | Label |
|---|---|
| 96 Well TC-Treated Microplates | NAME |
| Microplate | TYPE |
| Corning® | BRAND |
| CLS3799-1EA | SKU |
Fix the cells with 20µL (10% volume) paraformaldehyde 8% solution, resulting in a final concentration of 0.8% paraformaldehyde. Use a fume hood as this reagent is toxic.
Secure the lid and wrap plate edges with parafilm to prevent evaporation and store at 4°C prior to analysis (analyse within 48 hours, ideally later the same day).
Equipment
| Value | Label |
|---|---|
| Parafilm | NAME |
| lab consumable | TYPE |
| Parafilm M | BRAND |
| PM996 | SKU |
| Can be purchased from many vendors | SPECIFICATIONS |
Flow cytometry setup
Load plate into the flow cytometer, this protocol was developed using a CytoFLEX LX instrument.
Equipment
| Value | Label |
|---|---|
| CytoFLEX LX | NAME |
| Flow cytometer | TYPE |
| Beckman Coulter | BRAND |
| C40312 | SKU |
| CytoFLEX LX N3-V5-B3-Y5-R3-I2 Flow Cytometer (21 Detectors, 6 Lasers) | SPECIFICATIONS |
Set up the flow cytometer and run the daily Quality control (QC) protocol as per the instrument instructions.
In this case for the Cytoflex LX, multi-spectra beads
These beads may also be used to ensure consistency across experimental runs, as discussed in step 8.
Optimise gain and threshold settings for the detectors of interest in the flow cytometer. A spare aliquot of diatom culture can be used to optimise gain/thresold settings. Culture media obtained from filtering diatom culture (through a 0.22 μm filter) can also be used to identify background scatter and set thresholds.
The lazer and detectors are as follows, however this will depend on the particular instrument so exact numbers may not match if a different instrument is used.
Forward scatter area: Si-photodiode with built-in 488/8 band-pass filter (CytoFLEX Channel name: FSC-A)
Side scatter area: 488nm (50mw), Optical Filter: 488/8 nm, (CytoFLEX Channel name: SSC-A)
Chlorophyll a: Laser: 488nm (50mw), Optical Filter: 690/50 nm, (CytoFLEX Channel name: B690-50)
Thresholds were set at 4000 for FSC-A (forward scatter area) and 2000 for B690-50 (chlorophyll-a) to distinguish cells from background debris and particulates.
Flow cytometry analysis
Samples are mixed for 6 seconds by the CytoFlex instrument before analysis.
During development samples were generally run at medium speed/flow rate of 30µL per minute for 0h 1m 0s . Analysis is done on at least 200 cells, however in most cases it would be at least 2000 cells. The abort rate (%) was <1%. In the case of a highly dense culture, a slower speed maybe prudent to ensure a low abort rate and ensure accuracy of analysis. In the case of a low density culture, a higher flow rate or longer duration of analysis may be used to ensure an adequate number of cells are analyzed.
Diatoms were gated visuallt using event density plots of chlorophyll fluorescence and forward scatter (see below).
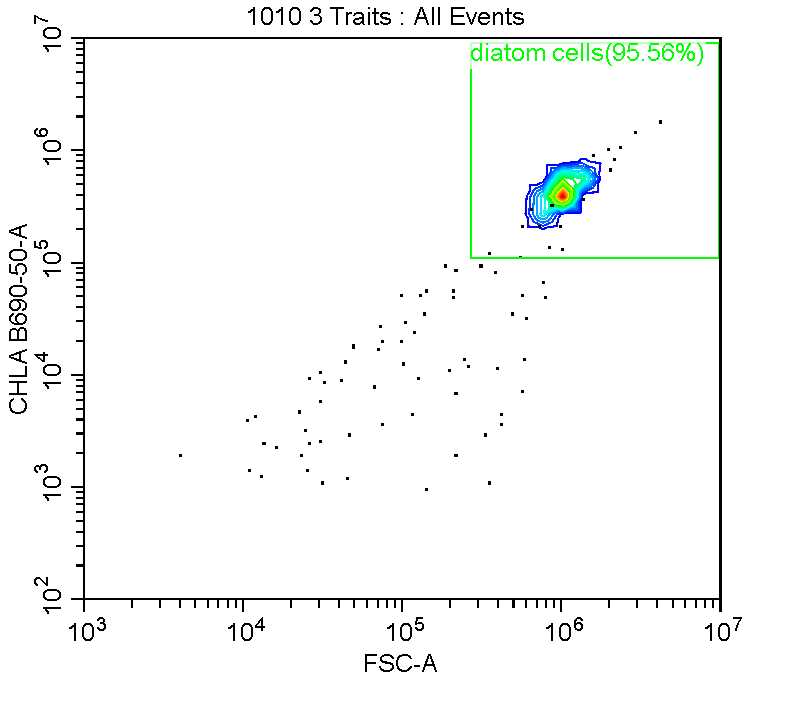
Calibration using beads
The multi-spectra beads
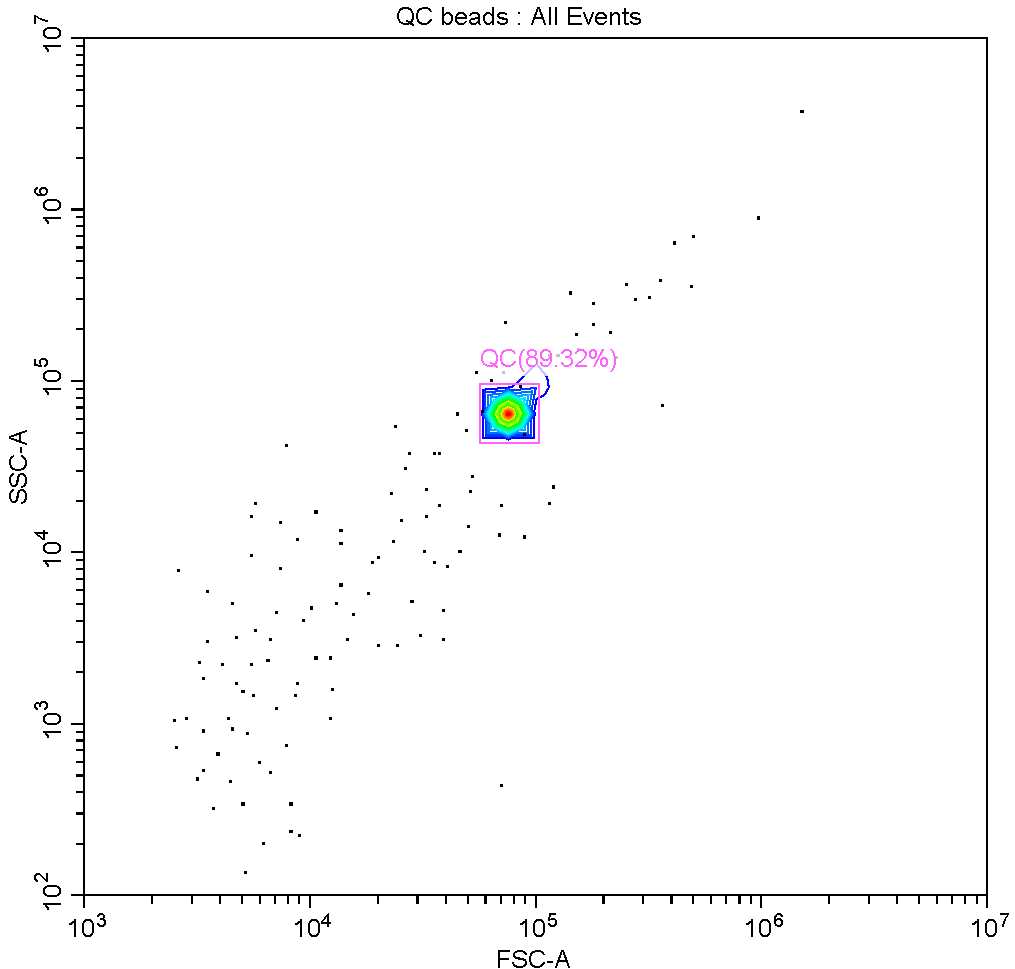
It is recommended to analyse samples from one experiment on the same day, however if this is not possible it is necessary to ensure that the instrument is performing similarly on the different days of analysis.
Using the parameters and thresholds established for diatoms, the QC beads may be run as if they were a sample and gated according to their chlorophyll fluorescence, forward scatter and side scatter (see image below). This way, if the beads fall within the same gates on each day of analysis, it may be assumed that samples run on different days may be fairly compared.
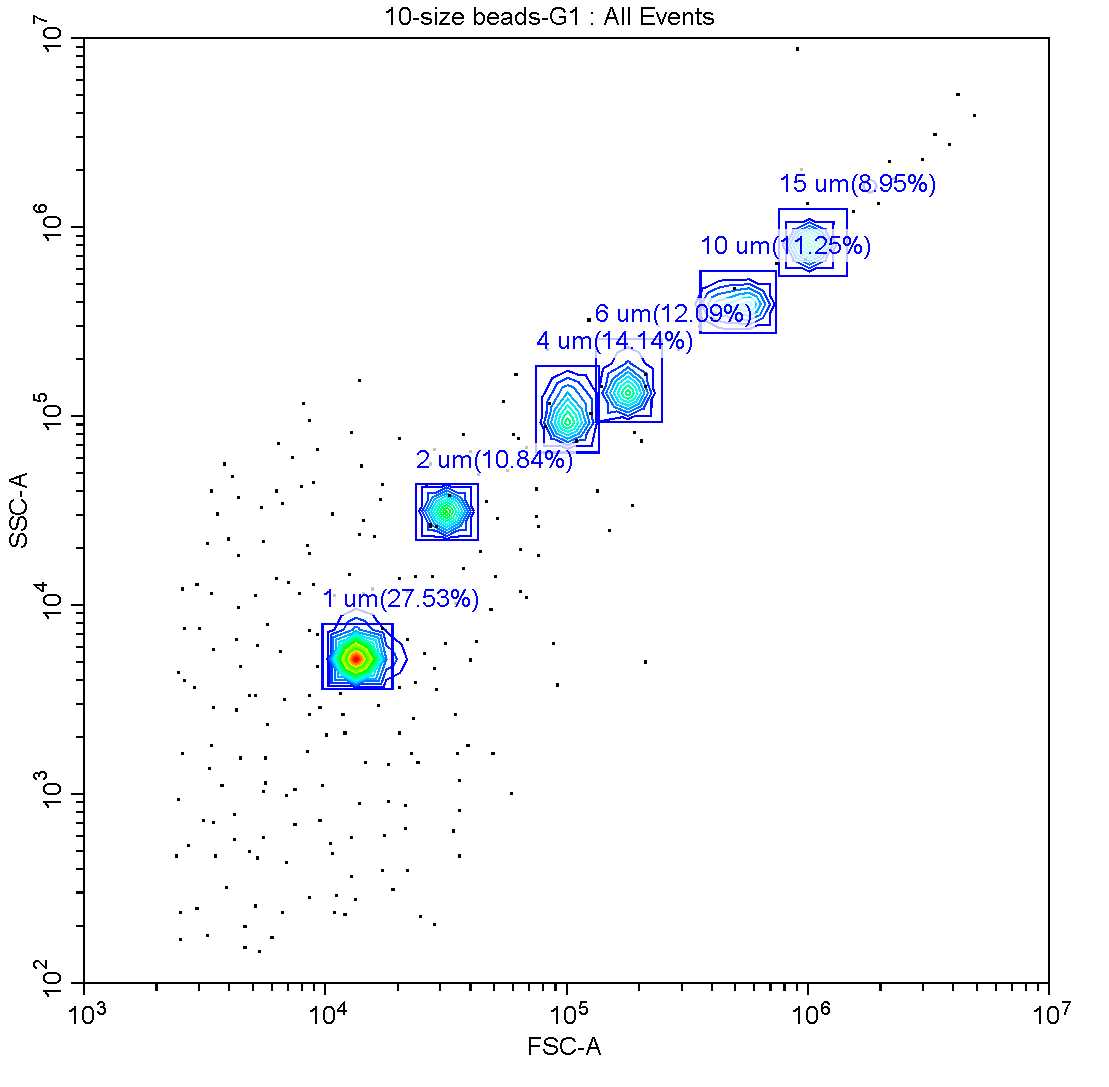
In addition to the QC beads, beads of known diameter were used to create a linear calibration of forward scatter vs. spherical size. For this protocol, beads of 2,4,6,10 and 15µm
Calculating cell size and metrics
Using the FSC measure of the size beads and the known diameters, create a linear equation to approximate spherical size of the diatom cells. Use the median FSC value based on a minimum of 200 cells but ideally 2000 or more.
e.g. $$ Spherical size = (FSC +275,549)/83,539
Spherical size may also be considered as "equivalent spherical diameter" or ESD, as the diatom cells are not actually spherical in shape.
For other metrics (granularity and chlorophyll-a), units are given as relative fluoresence units (RFU) from the flow cytometer, which can be used for comparative purposes between samples. Again, the median value is used from a minimum of 200 cells.
It is recommended that these values be corrected against cell size (ESD), as these traits are directly correlated with cell size, so it may be useful to assess these traits independent of size.

