Establishment and Maintenance of Organotypic Cerebellar Slice Cultures (OCerSC) from Aged Mice
Kaitlan S. Smith, Michael Fernandes de Almeida, Jonathan C Schisler
Disclaimer
NA.
Abstract
This protocol outlines a method for culturing organotypic slices from aged mouse cerebellar tissue, maintaining the cerebellum’s complex three-dimensional structure and cellular composition ex vivo. This technique simulates various physiological and pathophysiological conditions to study neurodevelopmental and neurodegenerative disorders and synaptic plasticity mechanisms. Expected outcomes include detailed neuronal morphology and functional connectivity analyses at the cellular and circuit levels using advanced imaging, electrophysiological techniques, and biochemical analyses such as protein expression, gene expression, metabolic activity, oxidative stress markers, neurotransmitter levels, calcium imaging, and enzyme activity assays.
Before start
Before you begin the slicing process, ensure that all six-well plates are prepped and placed in the incubator at 37 ºC with 5% CO2 for at least 30 minutes. This step is crucial to ensure that the media and inserts are at the correct temperature and conditions, which helps maintain the viability and quality of the cerebellum slices. Safety Precautions: * When handling the plates, incubator, animals, and equipment, wear appropriate personal protective equipment (PPE), such as gloves, safety eyewear, and a lab coat.
- Ensure that dissection is performed on a laboratory bench or in a separate hood dedicated to dissection.
- Use a different hood or area for cerebellum slicing to maintain a sterile environment.
- Follow proper aseptic techniques to minimize the risk of contamination and maintain sterility.
Steps
Materials
Slice Culture Consumables:
Tools:
- Scalpel
- Forceps
- Razor blade
- Scissors
- Transfer pipettes
- Paint brushes (recommended sizes no.3 and no.2)
Equipment
Equipment
| Value | Label |
|---|---|
| Biological safety cabinet | NAME |
| AC2-4S8-TU | TYPE |
| Esco | BRAND |
| 303110 | SKU |
Equipment
| Value | Label |
|---|---|
| Forma™ Series II Water-Jacketed CO2 Incubator, 184L | NAME |
| CO2 Incubators | TYPE |
| Thermo Scientific™ | BRAND |
| 3110 | SKU |
Equipment
| Value | Label |
|---|---|
| McIlwain Tissue Chopper with Petri Dish Modification | NAME |
| Tissue chopper | TYPE |
| Campden Instruments | BRAND |
| Model TC752-PD | SKU |
| https://campdeninstruments.com/ | LINK |
Reagents
Solutions
Slice Culture Media #1
Neurobasal-A Medium supplemented with:
- 2% B27
- 1% N2 Supplement
- 1% Glutamax
- 200 g/L glucose
- 1.5% Penicillin/streptomycin
- 1.5% Amphotericin B
- 80 μM Indomethacin
Slice Culture Media #2
Neurobasal-A Medium supplemented with:
- 2% B27
- 1% N2 Supplement
- 1% Glutamax
- 200 g/L glucose
- 1.5% Penicillin/streptomycin
- 1.5% Amphotericin B
Phosphate Buffered Saline
Preparation Steps
Before Starting Tissue Harvest and Slicing Steps
Only one cerebellum should be simultaneously sliced and harvested to minimize the time between cerebellum isolation and slice distribution to the inserted wells. This practice helps ensure the viability and quality of the cerebellum slices by reducing the time the tissue is exposed to non-optimal conditions.
Sterilize Equipment:
- Use 70% ethanol to wipe down all surfaces inside the biological safety cabinet thoroughly. Ensure all areas, including corners and hard-to-reach spots, are properly cleaned.
- Apply 70% ethanol to a clean cloth or paper towel and wipe down the tissue slicer and all dissection instruments, ensuring that all surfaces are clean.
- Allow the equipment to air dry completely before use.
Prepare Six-well Plates
- Place one cell culture insert per well and add 1mL of Slice Culture Media #1.
- Ensure membranes are thoroughly wet and that there are no air bubbles.
- Place the six-well plates in an incubator at
37°Cwith 5% CO2for at least 30 minutes before starting the cerebellar slicing process.
Prepare Tissue Slicer
- Mount a new blade on the tissue slicer.
- Set the slice thickness
250 µm.
Prepare the Chopping Disc
- Using Elmer’s glue, attach Whatman filter paper onto the chopping disc.
- Allow the glue to dry completely to ensure the filter paper is firmly attached.
- Mount the chopping disc onto the tissue slicer.
Prepare Hibernation Media Dishes
- Place Hibernate-A Medium in a
37°Cwater bath for at least 30 minutes before starting the cerebellar slicing process. Ensure the medium reaches the correct temperature to maintain tissue viability. - Prepare one 60 mm petri dish per animal.
- Add 4-5 mL of pre-warmed Hibernate-A Medium to each dish.
Prepare Euthanasia and Dissection Prep
- Set up the euthanasia chamber according to the approved animal protocol.
- Ensure that all necessary components are in place and functioning correctly.
- Double-check that the sterile dissection tools are ready.
- Obtain a 66 mm sterile petri dish for use during the dissection process.
- Prepare sterile PBS and set to chill on ice.
- Transport the mice to the dissection area. This protocol was developed using 22-92 week-old B6129F1/Tac mice.
Note
This protocol uses isoflurane to euthanize the animals. While other euthanasia methods, such as CO2, are feasible, isoflurane is recommended because it is less stressful for the animals and allows for the quick removal of the brain, ensuring that the tissue remains viable.
Cerebellar Slicing
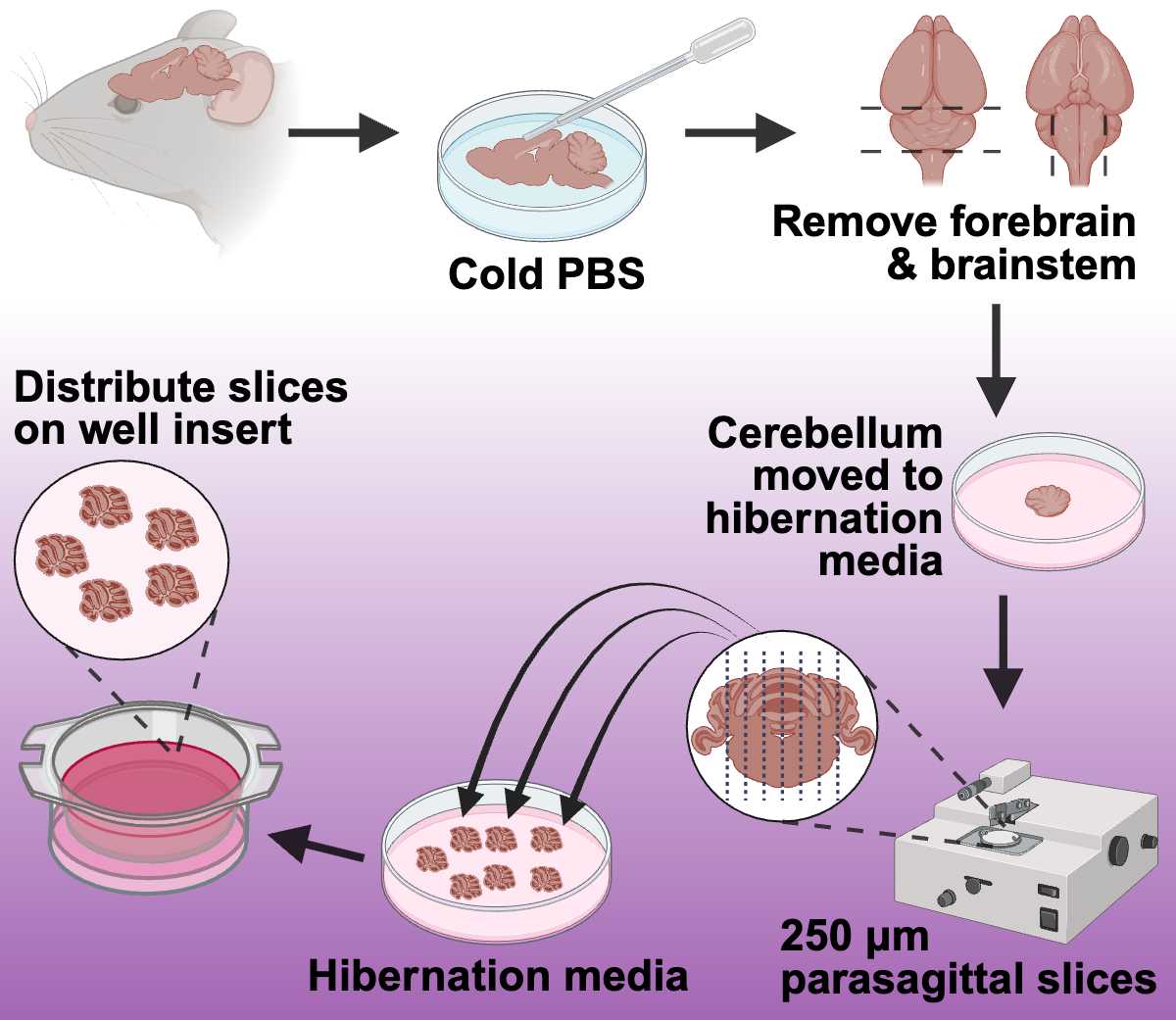
Dissect the Brain:
- Following proper IACUC-approved animal euthanasia, decapitate the animal.
- Carefully expose and remove the brain, taking special care not to damage the cerebellum.
- Transfer the brain to an empty sterile petri dish.
- Using a transfer pipette, wash the brain with cold, sterile PBS to remove any extraneous hair or blood ( Figure 1 ).
- Remove the forebrain and spinal cord to isolate the cerebellum ( Figure 1 ).
- Transfer the cerebellum to a sterile petri dish containing 4-5 ml of pre-warmed Hibernate media ( Figure 1 ).
Slicing the Cerebellum: 1. Transfer the cerebellum to the Whatman filter paper glued onto the chopping disc.
-
Critical Point: Orient the brain so the cerebellum faces upwards and the sagittal plane is aligned with the chopper blade ( Figure 1 ). This ensures that the slices will be sagittal, maintaining the structural integrity necessary for subsequent analyses.
-
Using the manual setting on the chopper, slice the cerebellum into
250 µmsections ( Figure 1 ). -
Use a paintbrush to hydrate the cerebellar tissue throughout the slicing process. (Avoid overwetting the cerebellum and the filter paper).
-
After each cut, use a paintbrush to transfer the cerebellum slice to a petri dish filled with pre-warmed Hibernate media ( Figure 1 ).
-
Select the intact cerebellar slices and transfer 4 to 6 individual slices onto the insert membranes in the six-well plates ( Figure 1 )to distribute them evenly on the insert.
-
Incubate at
37°Cwith 5% CO2.NoteWhen distributing the cerebellar slices onto the membranes, ensure that the slices are not close to the insert wall or touching one another. We recommend 4-6 slices per well for adult mice. Avoid the accumulation of media on the top of the membrane insert; if needed, remove excess medium carefully with a transfer pipette to secure the tissue's attachment to the insert membrane. A wet paintbrush can also be utilized to place the slices in an appropriate configuration following the removal of excess media.
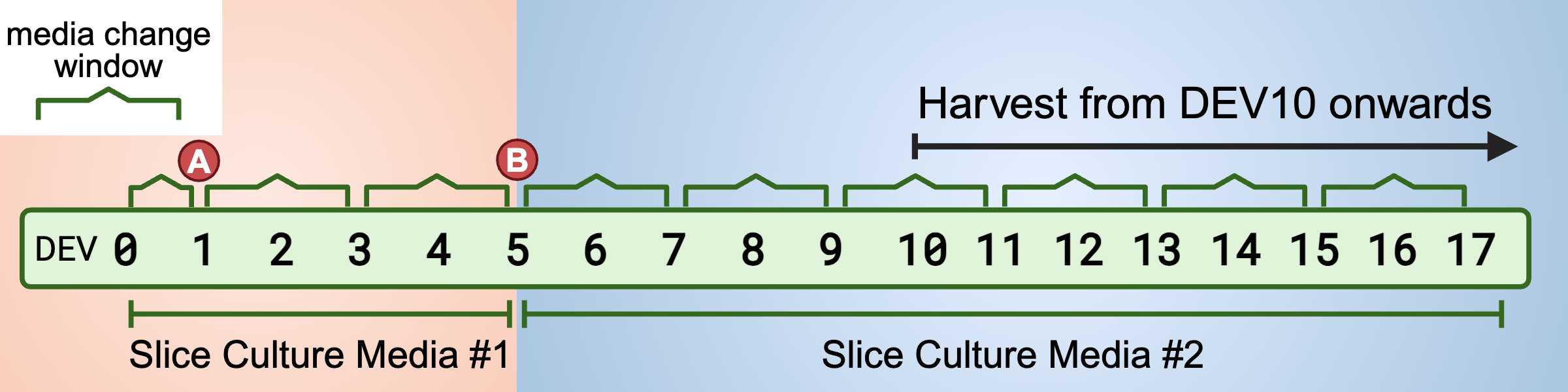
Culture Conditions
Incubation and Media Changes 1. Initial Incubation: Once slices are securely placed onto the membrane, incubate plates at 37ºC with 5% CO2. 37°C
- First Media Change (18-24 hours after slicing): Remove the slice culture media #1 and replace it with fresh slice culture media #1.
24h 0m 0s - Subsequent Media Changes: After the first media change, change the culture media every 2-3 days.
- Media Schedule:
- DEV 1-5: Maintain the OCerSC in Slice Culture Media #1, following a scheduled media change every 2-3 days.
48h 0m 0s - DEV6 - End of the experiment: Maintain the OCerSC in Slice Culture Media #2, with a scheduled media change every 2-3 days.
48h 0m 0s