Efficient third generation lentiviral particle production
Michelle Newbery, Simon Maksour, Amy Hulme, Neville S Ng, Mirella Dottori, Lezanne Ooi
Abstract
The overexpression of a gene of interest by third generation lentiviral particle generation systems is a critical process in molecular biology, cell biology and gene therapy research. While many lentiviral protocol production methods have been discussed in literature, this protocol takes into account previously established and novel optimisations to minimise user handling time, cost, and maximise practical yield. This protocol allows for at least 6 days of consecutive viral particle collection without compromising HEK293T cell culture or viral production efficiency, and can be easily and cost effectively reproduced in basic cell culture laboratories.
Steps
Lentiviral transfection
Maintain HEK293T cells in animal product free FreeStyle 293 Expression Medium or DMEM/F12 + GlutaMAX + HEPES + 5% FBS or 5% KSR. Include 50 U/mL Penicillin and Streptomycin to reduce risk of bacterial contamination if necessary.
Dissociate and subculture HEK293T at a density of >50000 cells/cm2 per transfer plasmid.
HEK293T cultures are inherently susceptible to detachment with acidification and may require weaning at high density for several passages to be sustained throughout the 6 day generation period. Viral particle production can be performed within at least 15 passages without loss of yield.
For each lentiviral transfer plasmid and packaging plasmid vectors, calculate reagent volumes required for 12 µg gene of interest vector, 4 µg pMDLg/pRRE (Addgene #12251), 4 µg pRSV-Rev (Addgene #12253) and 4 µg pCMV-VSV-G (Addgene #8454) and 60 µg PEI per 75 cm2of HEK293T culture.
Maximal transfection efficiency by PEI complex is typically obtained with higher proportions of PEI:DNA.
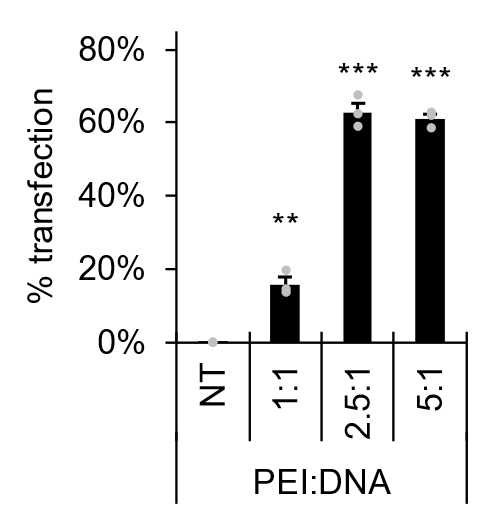
Prepare PEI-DNA solution in 1.5 mL of DMEM/F12 and incubate for 0h 5m 0s at ambient temperature.
Replace HEK293T cell culture medium with at least 0.2 mL/cm2 medium (e.g. 15-20 mL per 75 cm2 flask), add PEI-DNA complex solution, and return to incubator .
Note PEI-DNA complex transfection can occur without usage of low serum transfection medium products.
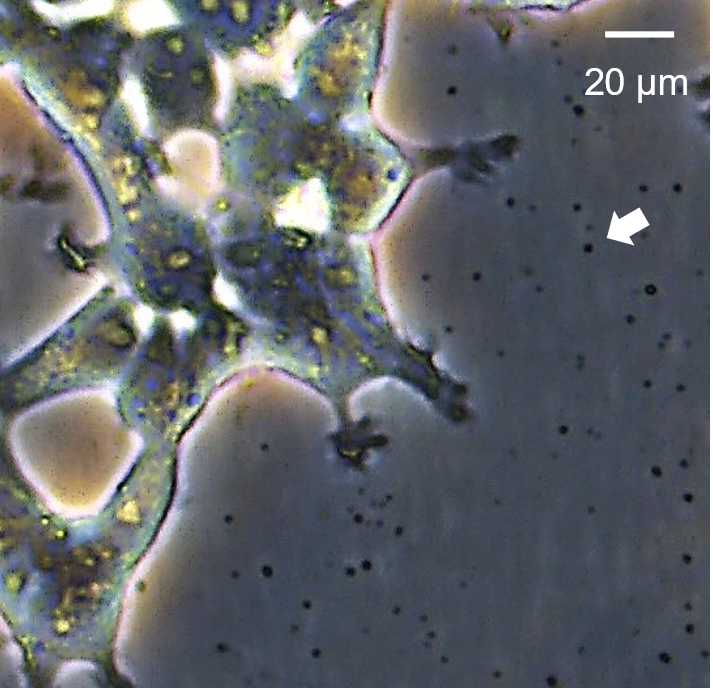
Each day collect medium in an appropriate sized sterile container (e.g. 120 mL specimen collection tubes), and store at 4°C. Proceed to viral particle concentration at selected endpoint.
We utilise viral particle concentration to avoid prolonged incubation of pluripotent or multipotent stem cells in HEK293T culture medium during viral transduction experiments. If viral particle concentration is not necessary, freeze immediately at -80°C.
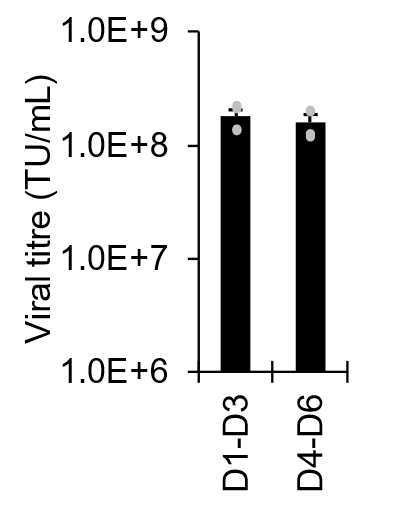
Lentiviral particle collection has been reported to be performed for at least up to 7 days (Rahman et al. 2013) and with multiple rounds of centrifugation (Ichim & Wells 2011). In this protocol we utilise a single centrifugation step performed on final day of collection on basis of finding negligible differences in loss of yield between day 3 and 6, which avoids multiple rounds of centrifugation (Figure 3).
Increase cell culture media volume per media change where necessary to reduce premature acidification, detachment and death. Additionally, cell culture vessels can be coated with PEI (0.005% w/v) or extracellular matrix such as collagen or Matrigel to reduce detachment.
Lentiviral particle concentration
Sterilise ultracentrifuge tubes and appropriate sized viral particle aliquot tubes (0.2 or 1.5 mL microcentrifuge tubes).
Optional: Filter supernatant through a 0.45 µm pore PES bottle top filter to remove cell debris. Alternatively, syringe filtration can be performed after lentiviral particle concentration.
Balance ultracentrifuge tubes within 0.1 g by weighing and adding appropriate amount of medium.
Collect lentiviral particles by centrifugation of supernatant at 50000x g,4°C.
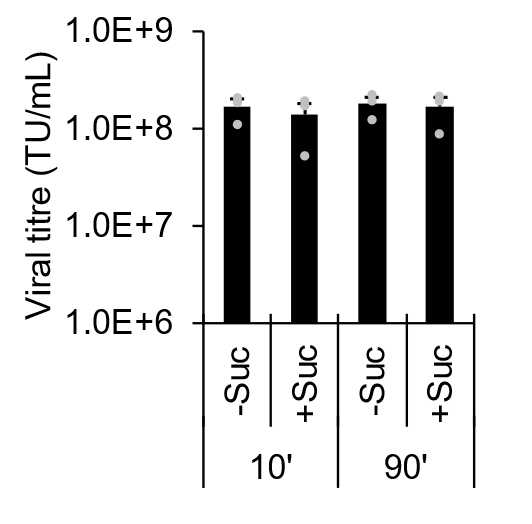
We have found routine centrifugation for ≥ 1.5 h can be truncated to as low as 10 minutes (Figure 4), however this should be confirmed per laboratory with consideration of ultracentrifugation equipment and tubes. Although prior studies have suggested sucrose layering may improve and titre (Jiang et al. 2015), we found no significant improvement of viral titre with a 10% sucrose cushion with either duration tested (Figure 4).
Carefully transfer tubes on ice, mark position of pellet and decant waste slowly to discard supernatant.
Resuspend pellet as a 200X concentrate of total supernatant (e.g. 200 µL from 40 mL of lentiviral supernatant).
If cell debris was not removed by filtration prior to concentration, centrifuge lentiviral concentrate at 1500x g, and discard pellet. Smaller debris can be removed with a 0.45 µm pore PES syringe filter if necessary.
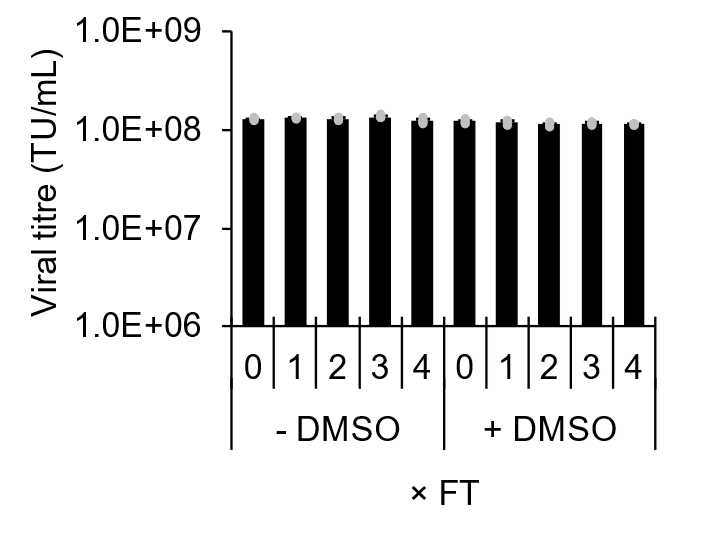
Prepare viral particle aliquots of appropriate volumes corresponding to application and freeze at -80°C. Lentivirus particles are enveloped and more susceptible to degradation in comparison to unenveloped DNA viruses, however resuspension in complete cell culture media is sufficient to prevent degradation during at least up to 4 freeze thaw cycles (Figure 5).
Viral titre can be calculated based on fluorescent reporter or immunofluorescent staining.
Lentiviral titre assay
Seed 10000 cells/well of intended cell type in a 96 well microplate allowing for at least 3-6 concentrations, 2-3 technical replicate wells per lentivirus and enumerating cell population, and return to incubator .
Prepare a 10-fold serial dilution of lentiviruses at intended concentrations (e.g. 1:1, 1:10, 1:100, or 1:1, 1:2, 1:10, 1:20, 1:100, 1:200), add to cell culture plate (e.g. 10 μL) and return to incubator.
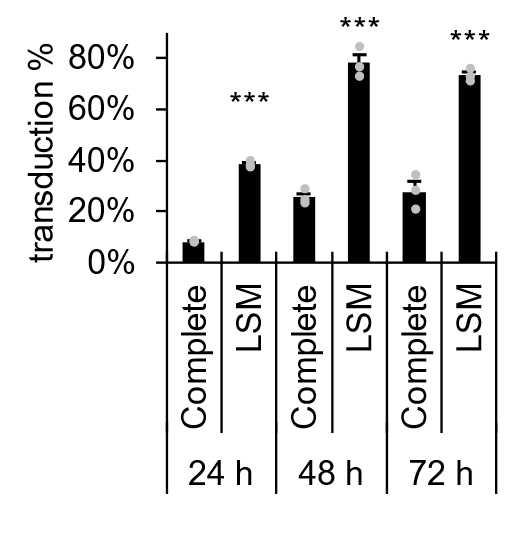
Optionally include an enhancer of transduction such as DEAE-Dextran or protamine sulfate at a concentration appropriate to cell type. We have found DEAE-Dextran (5-10 µg/mL) or protamine sulfate (50-100 µg/mL) effective at enhancing transduction in continuous cell lines and primary cells but ineffective in stem cells and neural precursor cells. Alternatively, we have found effective enhancement of transduction by replacement of medium in both continuous and stem cell lines with low serum medium (DMEM/F12+ITS-A+0.1% serum albumin) prior to addition of lentivirus (similar to Balak et al. 2019) (Figure 6), or by addition of lentivirus on same day as seeding in complete media.
On the same day as lentivirus addition, determine cell population in at least 2 wells.
Return to incubator .
The next day replace media, add transcriptional activator if applicable and return to incubator for 2-3 days.
Acquire microplate fluorescent microscopy images and score fluorescent reporter or immunolabelled gene of interest. Calculate functional titre based on wells with 5-40% transduction rate; TU/mL = (transduction %) multiplied by (cell population at time of transduction) divided by (inoculum volume in mL). Higher % transductions may underestimate titre due to increased rate of multiple integrations.
Please cite this protocol if used or adapted in any publications.

