Detection and Analysis of Proteins Modified by O-Linked N-Acetylglucosamine
Kamau Fahie, Kamau Fahie, Bhargavi Narayanan, Bhargavi Narayanan, Fiddia Zahra, Fiddia Zahra, Russell Reeves, Russell Reeves, Steve M. Fernandes, Steve M. Fernandes, Gerald W. Hart, Gerald W. Hart, Natasha E. Zachara, Natasha E. Zachara
Abstract
O-GlcNAc is a common post-translational modification of nuclear, mitochondrial, and cytoplasmic proteins that regulates normal physiology and the cell stress response. Dysregulation of O-GlcNAc cycling is implicated in the etiology of type II diabetes, heart failure, hypertension, and Alzheimer's disease, as well as cardioprotection. These protocols cover simple and comprehensive techniques for detecting proteins modified by O-GlcNAc and studying the enzymes that add or remove O-GlcNAc. © 2021 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Increasing the stoichiometry of O-GlcNAc on proteins before analysis
Basic Protocol 2 : Detection of proteins modified by O-GlcNAc using antibodies
Basic Protocol 3 : Detection of proteins modified by O-GlcNAc using the lectin sWGA
Support Protocol 1 : Control for O-linked glycosylation
Basic Protocol 4 : Detection and enrichment of proteins using WGA-agarose
Support Protocol 2 : Digestion of proteins with hexosaminidase
Alternate Protocol : Detection of proteins modified by O-GlcNAc using galactosyltransferase
Support Protocol 3 : Autogalactosylation of galactosyltransferase
Support Protocol 4 : Assay of galactosyltransferase activity
Basic Protocol 5 : Characterization of labeled glycans by β-elimination and chromatography
Basic Protocol 6 : Detection of O-GlcNAc in 96-well plates
Basic Protocol 7 : Assay for OGT activity
Support Protocol 5 : Desalting of O-GlcNAc transferase
Basic Protocol 8 : Assay for O-GlcNAcase activity
INTRODUCTION
The modification of Ser and Thr residues of nuclear, cytosolic, and mitochondrial proteins by O-linked β-N -acetylglucosamine (O-GlcNAc) is a dynamic post-translational modification of metazoans. Although O-GlcNAc has been identified in all metazoans studied, recent data suggest that plants use both O-fucose and O-GlcNAc (Xu, Chalkley, Wang, & Burlingame, 2012; Zentella et al., 2017), protozoans use either O-GlcNAc or O-fucose (Banerjee, Robbins, & Samuelson, 2009), and that while filamentous yeast use O-GlcNAc (Schindler, Hogan, Miller, & DeGaetano, 1987), Saccharomyces cerevisiae may use mannose (Halim et al., 2015).
O-GlcNAc is cycled on and off proteins by two dedicated enzymes: the O-GlcNAc transferase (OGT) that catalyzes the addition of O-GlcNAc and the O-GlcNAcase (OGA) that catalyzes the removal of O-GlcNAc. The reversible nature of the O-GlcNAc modification allows multiple rounds of regulation and alteration of function to occur over the lifetime of proteins. Indeed, protein O-GlcNAcylation has been demonstrated to influence protein function by altering structure and stability (Brister, Pandey, Bielska, & Zondlo, 2014; Elbaum & Zondlo, 2014; Levine et al., 2019; Yuzwa et al., 2012), enzymatic activity (Han et al., 2017; Rao et al., 2015; Yi et al., 2012), protein-protein interactions (Toleman et al., 2018), and post-translational modifications (Chou, Hart, & Dang, 1995; Comer & Hart, 2001). O-GlcNAcylation is responsive to stimuli/signaling including nutrient availability, cellular stress, insulin stimulation, or cellular stages such as those of the cell cycle, suggesting that cells use O-GlcNAc to remodel cellular pathways (Hart, Slawson, Ramirez-Correa, & Lagerlof, 2011).
Proteomic studies suggest that more than 4000 proteins of diverse function are modified by O-GlcNAc. Proteins modified by O-GlcNAc include cytoskeletal proteins, nuclear pore proteins, RNA polymerase II (RNA Pol II), transcription factors, proto-oncogene products, tumor suppressors, hormone receptors, phosphatases, and kinases (Chalkley, Thalhammer, Schoepfer, & Burlingame, 2009; Khidekel et al., 2007; Nandi et al., 2006; Trinidad et al., 2012; Vosseller et al., 2006). Not surprisingly, O-GlcNAc has been implicated in regulating key cellular pathways, including epigenetics, transcription, translation, protein homeostasis, autophagy, nutrient sensing and metabolism, immune signaling, and the cell cycle. Moreover, perturbations in the metabolism of UDP-GlcNAc, which can alter the regulation of many O-GlcNAc-modified proteins, have been implicated in Alzheimer's disease, diabetes, and cancer (Hart et al., 2011; Ma & Vosseller, 2014; Zhu, Shan, Yuzwa, & Vocadlo, 2014).
These protocols concentrate on techniques for the detection and analysis of proteins modified by O-GlcNAc, as well as methods for the analysis of enzymes responsible for O-GlcNAc addition and removal. The major focus is on procedures that require standard laboratory equipment. First, a protocol for increasing the stoichiometry of O-GlcNAc on proteins is given (see Basic Protocol 1). This is followed by simple techniques for the detection and screening of O-GlcNAc-modified proteins either by immunoblotting or by lectin affinity chromatography (see Basic Protocols 2-5). Separate procedures are described for verifying that the glycan is O-linked GlcNAc (see Support Protocols 1 and 2). These are followed by protocols for a more comprehensive analysis of O-GlcNAc-modified proteins, including labeling of O-GlcNAc residues with [3H]Gal, and subsequent product analysis (see Alternate Protocol, Basic Protocol 5, and Support Protocols 3 and 4). Also described is a method that enables the detection of O-GlcNAc in 96-well plate format (see Basic Protocol 6). The final set of protocols provide assays for O-GlcNAc transferase and O-GlcNAcase activity, respectively, along with a method to desalt O-GlcNAc transferase (see Basic Protocols 7 and 8 and Support Protocol 5).
Basic Protocol 1: INCREASING THE STOICHIOMETRY OF O-GlcNAc ON PROTEINS BEFORE ANALYSIS
In cultured mammalian cells, as well as tissue slices and tissues in vivo, the stoichiometry of O-GlcNAc moieties per protein molecule can be increased by treating cells/tissues/animals with inhibitors of O-GlcNAcase. Several inhibitors exist (Dennis et al., 2006; Kim, Kang, Love, & Hanover, 2006; Macauley, Whitworth, Debowski, Chin, & Vocadlo, 2005; Shanmugasundaram et al., 2006; Yuzwa et al., 2008), although only PUGNAc (Toronto Research Chemicals; Haltiwanger, 1998) and Thiamet-G (Cayman Chemicals; Yuzwa et al., 2008) are commercially available. Unlike Thiamet-G, PUGNAc also inhibits lysosomal hexosaminidases. Several OGT inhibitors have been developed (Gross, Kraybill, & Walker, 2005). Although these initial inhibitors were demonstrated to work in neonatal cardiomyocytes (1-5 μM; Ngoh et al., 2008) and breast cancer cells (500 μM; Caldwell et al., 2010), recent data suggests that they react with the protein backbone, limiting their utility (Jiang, Lazarus, Pasquina, Sliz, & Walker, 2011). Recently, high-throughput screening led to the development of a small-molecule OGT inhibitor, OSMI-1, that is cell permeable (Ortiz-Meoz et al., 2015). Further structure-based evolution of OSMI-1 yielded three additional compounds, OSMI-2, -3, and -4, with higher affinities for OGT (Martin et al., 2018). One alternative strategy has been to use cell-permeable compounds that are metabolized by the cell to generate non-hydrolyzable analogs of UDP-GlcNAc. Examples include Ac45SGlcNAc (effective in cells; Gloster et al., 2011) or 5SGlcNHex (effective in animals; Liu et al., 2018). However, it is important to note that OGT inhibitors that mimic UDP-GlcNAc are likely to affect other enzymes that utilize UDP-GlcNAc as a substrate. Streptozotocin (STZ; Roos et al., 1998), glucosamine (Han, Oh, & Kudlow, 2000), and the glutamine fructose-6-phosphate amidotransferase (GFAT) inhibitors 6-diazo-5-oxonorleucine (DON) and azaserine have also been used to alter the stoichiometry of O-GlcNAc on proteins. However, STZ has been shown to induce poly(ADP-ribose) polymerase–mediated apoptosis in Min6 cells (Gao, Parker, & Hart, 2000) and should be used with caution. STZ and glucosamine treatments are most effective in cells that express the glucose transporter GLUT-2 (Schnedl, Ferber, Johnson, & Newgard, 1994). Notably, inhibitors of GFAT can have off-target effects on other glutamine-utilizing enzymes. Benzyl-α-GalNAc and benzyl-β-GlcNAc, which have been reported to inhibit OGT, should be avoided, as these have been characterized as inhibitors of prototypical O-GalNAc-type glycosylation. Care should be taken when using inhibitors of OGT and OGA, as treatment at too high a dose or for too long can affect the maturation of the mRNAs encoding OGT and OGA and thus protein expression (Park et al., 2017; Tan et al., 2020).
Materials
-
Cells of interest growing in monolayer culture in 100-mm dishes, and appropriate culture medium
-
O-GlcNAcase inhibitor stock:
- 1 mM Thiamet-G (Millipore-Sigma; SML0244) in 1 M HEPES, pH 7.5 (filter sterilize and store in aliquots up to 6-12 months at −80°C);
- 100 mM PUGNAc (Millipore-Sigma; A7229) in PBS, pH 7.4 (filter sterilize and store in aliquots up to 6-12 months at −80°C); or
- 500 mM glucosamine in 500 mM HEPES, pH 7.5 (make just before use; filter sterilize)
-
Ice
-
100-mm tissue culture dishes
-
Humidified water-jacket CO2 incubator
-
Additional reagents and equipment for SDS-PAGE and electroblotting (Gallagher, 2001)
1.Grow cells in monolayer culture in a sufficient number of 100-mm dishes.
2.Add (or replace) growth medium with fresh medium containing 0.1-1 μM Thiamet-G (added from 1 mM stock), 10-100 μM PUGNAc (added from 100 mM stock), or 5 mM glucosamine (added from 500 mM stock). Incubate the cells in a humidified water-jacket CO2 incubator at 37°C for 4-18 hr.
3.At the end of treatment, take the dishes out of the incubator and place on ice. Extract as desired. Separate proteins by SDS-PAGE and electroblot onto an appropriate membrane (Gallagher, 2001).
Basic Protocol 2: DETECTION OF PROTEINS MODIFIED BY O-GlcNAc USING ANTIBODIES
Several antibodies have been developed that recognize terminal GlcNAc residues or the O-GlcNAc modification (Table 1). Additionally, site-specific O-GlcNAc antibodies have been raised against glycosylation sites on proteins including c-Myc (Kamemura, Hayes, Comer, & Hart, 2002), tau (Cameron et al., 2013; Yuzwa et al., 2011), SirT1 (Shan et al., 2018), and CRMP2 (Muha et al., 2019), as well as IRS2 and a subset of histones (H2A, H2B, H3, H4; available from GlycoScientific). These tools allow researchers to probe the O-GlcNAc modification state of key proteins without first purifying the protein. Site-specific antibodies are not discussed further because they are not widely available. When using O-GlcNAc pan-specific antibodies (antibodies that recognize O-GlcNAc linked to Ser/Thr residues) or GlcNAc pan-specific antibodies (antibodies that recognize any terminal β-GlcNAc residue, including those linked to longer glycan structures), it is important to run appropriate controls, as some antibodies cross-react with peptide sequences that mimic GlcNAc residues (Shikhman, Greenspan, & Cunningham, 1994), whereas others have some dependence on the peptide backbone (Holt et al., 1987; Snow, Senior, & Gerace, 1987). Appropriate controls include: (1) elevating O-GlcNAc levels in tissue culture using inhibitors of the O-GlcNAcase or glucosamine (see Basic Protocol 1); (2) lowering O-GlcNAcylation in tissue culture cells using inhibitors of OGT or the hexosamine biosynthetic pathway; (3) removing O-GlcNAc from samples in vitro using hexosaminidase (see Support Protocol 3); (4) elevating the levels of O-GlcNAc in tissue culture by overexpressing OGT or performing RNA interference (RNAi) of O-GlcNAcase; (5) lowering O-GlcNAcylation by overexpressing O-GlcNAcase or performing RNAi of OGT; (6) treating lysates with PNGase F to remove N-linked glycans; (7) using appropriate purified control proteins, such as ovalbumin, which bears N-linked glycans with terminal GlcNAc residues, or synthetic neoglycoconjugates, such as BSA-aminophenyl-GlcNAc (BSA-AP-GlcNAc); or (8) competing away antibody binding with free GlcNAc that controls for nonspecific binding and cross-reactivity with endogenous immunoglobulin.
| Name | Antibody isotype | Recognizes | Commercially available | Positive control | Negative control | Reference |
|---|---|---|---|---|---|---|
| CTD110.6 | IgM | βGlcNAc | Covance, Pierce, Millipore Sigma, Cell Signaling, Santa Cruz Biotechnology | BSA-AP-GlcNAc | BSA-APa or ovalbumin | Comer et al. (2001) |
| RL2 | IgG | O-GlcNAc | Abcam, Affinity Bioreagents, Santa Cruz Biotechnology | BSA-AP-GlcNAc | BSA-AP or ovalbumin | Snow et al. (1987) |
| MY95 | IgG | O-GlcNAc | No | BSA-AP-GlcNAc | BSA-AP or ovalbumin | Matsuoka, Matsuoka, Shibata, Yasuhara, and Yoneda (2002) |
| 18B10.C7(3) | IgG | O-GlcNAc | Millipore | BSA-AP-GlcNAc | BSA-AP or ovalbumin | Teo et al. (2010) |
| 9D1.E4(10) | IgG | βGlcNAc | Millipore | BSA-AP-GlcNAc | BSA-AP or ovalbumin | Teo et al. (2010) |
| 1F5.D6(14) | IgG | O-GlcNAc | Millipore | BSA-AP-GlcNAc | BSA-AP or ovalbumin | Teo et al. (2010) |
| HGAC 85 | IgG | βGlcNAc | Novus Biologicals, Abcam, Pierce, Enzo Life Sciences, Affinity Bioreagents | BSA-AP-GlcNAc | BSA-AP or BSA | Turner, Tartakoff, and Greenspan (1990) |
| HGAC 49 | IgM | βGlcNAc | No | BSA-AP-GlcNAc | BSA-AP or BSA | Turner et al. (1990) |
| HGAC 39 | IgG | βGlcNAc | No | BSA-AP-GlcNAc | BSA-AP or BSA | Turner et al. (1990) |
| Multi-mAbb | IgG | O-GlcNAc | Cell Signaling Technology | BSA | N/A |
- a
BSA-AP, BSA-aminophenol.
- b
This antibody was raised against glycoproteins. Modulating O-GlcNAc levels is an appropriate control.
Using the Millipore Sigma Immobilon Western Enhanced Chemiluminescent (ECL) Horseradish peroxidase (HRP) Substrate system, the authors have found that 10 μg of nuclear or total cell extract is sufficient. However, 20-30 μg of cell extract provides the highest quality data. For the detection of O-GlcNAc from tissue samples, the authors have established that 25-30 μg of total tissue lysate is required for detection using CTD110.6.For detection using the RL2 antibody, 15-20 μg of tissue lysate is sufficient to be within the linear range of detection using the aforementioned ECL system. For purified proteins, Comer and co-workers found that 25-50 ng of a neoglycoconjugate was sufficient (Comer, Vosseller, Wells, Accavitti, & Hart, 2001). The protocol given is for CTD110.6 (Comer et al., 2001), which appears to have the least peptide dependence and recognize the greatest number of O-GlcNAc-modified proteins. A table summarizing conditions for a subset of commercial antibodies is included; the protocols are similar to that for CTD110.6 (Table 2).
| Primary antibody | Blocka | Primary concentration | Primary buffer | Secondary antibody | Secondary buffera | GlcNAc competition |
|---|---|---|---|---|---|---|
| CTD110.6 | 3% milk | 1 μg/ml | 3% BSA | Mouse anti-IgM | 3% BSA | 100 mM |
| RL2 | 3% milk | 1 μg/ml | 3% BSA | Mouse anti-IgG | 3% BSA | 500 mM |
| HGAC 85 | 3% BSA | 1 μg/ml | 3% BSA | Mouse anti-IgG | 3% BSA | 250 mM |
| HGAC 49 | 3% BSA | 1 μg/ml | 3% BSA | Mouse anti-IgM | 3% BSA | 250 mM |
| HGAC 39 | 3% BSA | 1 μg/ml | 3% BSA | Mouse anti-IgG | 3% BSA | 250 mM |
| Multi-mAb (CST)b | 3% milk | 1 μg/ml | 3% BSA | Rabbit anti-IgG | 3% BSA | 250 mM |
- a
Milk and BSA are (w/v) and are dissolved in TBST.
- b
CST, Cell Signaling Technologies.
IMPORTANT NOTE : Volumes are given for 9 × 14-cm membranes washed in 10.5 × 15.5-cm boxes. Volumes can be scaled down or up, but membranes should be covered.
Materials
-
Purified or crude protein (e.g., Basic Protocol 1) separated by SDS-PAGE and electroblotted to polyvinylidene difluoride (PVDF; Gallagher, 2001) or nitrocellulose (preferred to PVDF) (duplicate blots are required)
-
Blocking buffer: 3% (w/v) milk in TBST (see recipe for TBST)
-
TBST (see recipe)
-
Antibody: CTD110.6 (Millipore Sigma, MABS1254) diluted to 1 μg/ml in antibody dilution buffer (3% BSA w/v in TBST)
-
N -Acetylglucosamine (GlcNAc; Millipore Sigma, A4106)
-
HRP-conjugated anti-mouse IgM (Millipore Sigma, A8786) diluted 1/5000 in antibody dilution buffer (3% BSA [w/v] in TBST)
-
TBS (see recipe)
-
Immobilon Western Chemiluminescence HRP Substrate (Millipore Sigma, WBKLS0500)
-
Additional reagents and equipment for visualization with chromogenic and luminescent substrates (Gallagher, 2001)
1.Block the blots by incubating with 25 ml blocking buffer for 60 min at room temperature.
2.Wash the blots with 25 ml TBST three times, each time for 10 min at room temperature.
3.Incubate the blots with CTD110.6 (diluted to 1 μg/ml in antibody dilution buffer), in duplicate, with and without 100 mM GlcNAc, overnight at 4°C.
4.Wash the blots with 25 ml TBST three times, each time for 10 min at room temperature.
5.Incubate the blots with HRP-conjugated anti-mouse IgM (1/5000 dilution in antibody dilution buffer) for 50 min at room temperature.
6.Wash the blots with 25 ml TBST four times, each time for 10 min at room temperature.
7.Wash the blots with 25 ml TBS once, for 10 min at room temperature.
8.Develop the HRP reaction using, e.g., the ECL system (Gallagher, 2001).
Basic Protocol 3: DETECTION OF PROTEINS MODIFIED BY O-GlcNAc USING THE LECTIN sWGA
Many lectins are reportedly specific for β-GlcNAc residues. The authors have typically used succinylated wheat germ agglutinin (sWGA), which is widely available and is derivatized with a number of useful functional groups including HRP. Before succinylation, WGA will recognize both sialic acid and GlcNAc (Monsigny et al., 1979). For additional information concerning lectin chromatography, see Freeze (2001).
The amount of “test” protein used is dependent on the technique(s) used to develop the HRP reaction. Using the Millipore Sigma Immobilon Western Enhanced Chemiluminescent HRP Substrate system, the authors find that 15 μg of cytoplasmic or nuclear extract is sufficient, but 20-30 μg of protein produces the best data.
It is important to include an appropriate positive (100 ng ovalbumin) and negative (100 ng BSA) control. As a control, a portion of the sample should also be treated with hexosaminidase (see Support Protocol 2), to show that reactivity is toward GlcNAc. Alternatively, the levels of O-GlcNAc can be manipulated in cell culture (see Basic Protocol 1). An additional control is to subject the blot to mild reductive β-elimination to verify that lectin/antibody reactivity is towards O-linked glycans (see Support Protocol 1), rather than N-linked glycans.
IMPORTANT NOTE : Volumes are given for 9 × 14-cm membranes washed in 10.5 × 15.5-cm boxes. Volumes can be scaled down or up, but membranes should be covered.
Materials
-
Purified or crude protein separated by SDS-PAGE and electroblotted (Gallagher, 2001) to polyvinylidene difluoride (PVDF) or nitrocellulose (duplicate blots are needed.)
-
5% (w/v) BSA in TBST (see recipe for TBST)
-
TBST (see recipe)
-
0.1 μg/ml HRP-conjugated sWGA (EY Labs; H-2102) in TBST: the lectin can be stored at 1 mg/ml in 0.01 M PBS, pH 7.4 (Moore, 2001), for at least 1 year at −20°C
-
N -Acetylglucosamine (GlcNAc; Millipore Sigma; A4106)
-
High-salt TBST (HS-TBST): TBST containing 1 M NaCl
-
Tris-buffered saline (TBS; see recipe)
-
ECL kit (Millipore Sigma Immobilon Western Chemiluminescent HRP Substrate, WBKLS0500)
-
Additional reagents and equipment for visualization with chromogenic and luminescent substrates (Gallagher, 2001)
1.Wash the duplicate blots for 10 min in 25 ml TBST at room temperature.
2.Block by incubating the blots in 25 ml 5% (w/v) BSA/TBST for at least 60 min at room temperature.
3.Wash the blots three times, each time for 10 min with 25 ml TBST at room temperature.
4.Incubate the blots in 0.1 μg/ml sWGA-HRP in HS-TBST, in duplicate, with and without 1 M GlcNAc, overnight at 4°C.
5.Wash the blots six times, each time for 10 min, with 25 ml HS-TBST at room temperature.
6.Wash the blots once with 25 ml TBS for 10 min at room temperature.
7.Develop the HRP-reaction using, e.g., the ECL system (Gallagher, 2001).
Support Protocol 1: CONTROL FOR O-LINKED GLYCOSYLATION
Traditionally, mild alkaline reduction (reductive β-elimination) has been used to release O-linked carbohydrates from proteins (Amano & Kobata, 1989). This method has been adapted for blots to show that lectin/antibody reactivity is toward O-linked rather than N-linked glycans (Duk, Ugorski, & Lisowska, 1997). Proteins blotted to PVDF are treated with 55 mM NaOH overnight (releasing O-linked sugars) and then probed using lectins or antibodies (Reeves, Lee, Henry, & Zachara, 2014). The authors note that this protocol will not differentiate between O-GlcNAc and other forms of O-linked glycosylation.
There are a number of reasons why lectin/antibody reactivity could be lost after NaOH treatment, e.g., the sugars were destroyed instead of being released, or the protein was degraded. To control for these, it is important to have control proteins with N- and O-linked sugars, and to stain one blot for protein after treatment, preferably with an antibody. The authors suggest a control blot of bovine asialofetuin (Millipore Sigma), which contains both N- and O-linked sugars terminating in GlcNAc, treated and not treated with PNGase F (Powell, 2001).
IMPORTANT NOTE : Volumes are given for 9 × 14-cm membranes washed in 10.5 × 15.5-cm boxes. Volumes can be scaled down or up, but membranes should be covered.
Materials
-
Protein samples and controls blotted to PVDF (triplicate blots are needed; nitrocellulose is not suitable as it dissolves in 55 mM NaOH)
-
Tris-buffered saline (TBS; see recipe)
-
55 mM NaOH
-
Milli-Q water
-
TBST (see recipe)
-
3% (w/v) BSA in TBST (see recipe for TBST)
-
40°C water bath
-
Additional reagents and equipment for probing protein blots with protein-specific antibodies (see Basic Protocol 2) or lectins (see Basic Protocol 3)
1.Wash the blots once with 25 ml TBS for 10 min at room temperature.
2.Incubate the two blots in 25 ml 55 mM NaOH at 40°C overnight; incubate the control blot in 25 ml Milli-Q water at 40°C overnight.
3.Wash the blots three times, each time for 10 min at room temperature, with 25 ml TBST.
4.Block by incubating the blots in 25 ml of 3% (w/v) BSA/TBST for 60 min at room temperature.
5.Probe the blots (one treated and one untreated) with carbohydrate-specific lectins (see Basic Protocol 3) or antibodies (see Basic Protocol 2). Probe the second NaOH-treated blot with a protein-specific antibody (see Basic Protocol 2).
Basic Protocol 4: DETECTION AND ENRICHMENT OF PROTEINS USING WGA-AGAROSE
WGA lectin affinity chromatography provides a convenient method for enriching and detecting O-GlcNAc-modified proteins. This procedure has been adapted for detecting proteins that are difficult to purify or are present in low copy numbers, such as transcription factors. In this protocol, the protein of interest is synthesized in a rabbit reticulocyte lysate (RRL) in vitro transcription-translation (TNT) system (Promega) and labeled with either [35S]Met, [35S]Cys, or [14C]Leu. After desalting, the proteins are tested for their ability to bind WGA-agarose in a GlcNAc-specific manner (Roquemore, Chou, & Hart, 1994). This protocol is readily adapted to purifying proteins from cell extracts, but, as WGA binds proteins with both terminal GlcNAc and sialic acid residues, typically one would purify proteins from nuclear and cytoplasmic extracts to avoid co-purifying proteins with prototypical glycans.
Alternatively, the lectin Ricinus communis agglutinin 1 (RCA1) has been used to select for O-GlcNAc proteins that have previously been labeled by galactosyltransferase (see Alternate Protocol). Proteins modified by terminal Gal are specifically retained on an RCA1 affinity column. Labeled O-GlcNAc-modified proteins are released under mild conditions, whereas those containing N-linked structures require lactose addition to the buffer before elution results (Greis & Hart, 1998; Hayes, Greis, & Hart, 1995). The method described in this protocol can be adapted for RCA1 affinity chromatography by substituting RCA1-agarose (Vector Laboratories) for WGA-agarose and changing the order of the Gal and GlcNAc elution buffer. In choosing a label to use, ensure that the labeled amino acid is well represented in your protein of interest, although [35S]Met is usually the most cost-effective and sensitive choice.
Materials
-
cDNA subcloned into an expression vector with an SP6 or T7 promoter (∼0.5-1 μg/μl)
-
TNT Lysate Systems kit (Promega)
-
Label: [35S]Met, [35S]Cys, or [14C]Leu
-
WGA-agarose (Vector Laboratories; AL1023)
-
WGA wash buffer: PBS (Moore, 2001) containing 0.2% (v/v) Nonidet P-40 (NP-40)
-
WGA Gal elution buffer (see recipe)
-
WGA GlcNAc elution buffer (see recipe)
-
TCA or methanol
-
1-ml tuberculin syringe with glass wool plug at the bottom to support chromatography matrix or Bio-Rad Bio-Spin disposable chromatography column
-
Liquid scintillation counter
-
Additional reagents and equipment for digesting proteins with hexosaminidase (see Support Protocol 2), desalting (see Support Protocol 5), and SDS-PAGE and autoradiography (Gallagher, 2001)
Prepare the proteins
1.Synthesize the proteins to incorporate the desired label ([35S]Met, [35S]Cys, or [14C]Leu) using the TNT system according to the manufacturer's instructions. Include the protein of interest, a positive control for WGA binding (e.g., the nuclear pore protein p62), a negative control (luciferase, supplied with kit), and a no-DNA control.
2.Treat half of each sample with hexosaminidase (see Support Protocol 2).
3.Desalt the samples using spin filtration (e.g., Microspin G-50 columns, Cytiva Life Sciences) or a 1-ml G-50 desalting column (as for desalting O-GlcNAc transferase; see Support Protocol 5).
Apply the protein samples to chromatography columns
The following procedure is carried out at 4°C.
4.Equilibrate WGA-agarose and pack column as follows:
- If the resin is supplied as 50% slurry (i.e., 50% resin/50% storage solution), remove 300 μl (double the volume required) and pipet into a 1-ml tuberculin syringe or disposable chromatography column.
WGA is used here rather than sWGA as it has a higher affinity for GlcNAc and O-GlcNAc.
-
Let the storage solution drain from resin.
-
Equilibrate resin by washing column four times, each time with 1 ml WGA wash buffer. Cap the column.
The volumes given are appropriate for a sample derived from a TNT reaction. For enrichment of other protein samples, the volume of WGA should be optimized for the protein sample applied. The authors find that 50 mg of cell extract requires 5 ml of WGA-agarose, assuming that 1%-2% of the total cell extract is modified by O-GlcNAc.
5.Apply the sample (∼30 μl of an TNT reaction) to the column and let stand at 4°C for 30 min, or cap and incubate at 4°C for 30 min with rotating or rocking.
Wash the column and elute GlcNAc
6.At the end of the 30-min incubation, uncap the column and allow the sample to “run through” the resin. Collect this as the “run through” fraction. Wash the column with 15 ml WGA wash buffer at 10 ml/hr, collecting 0.5-ml fractions.
7.Load the column with 300 μl WGA Gal elution buffer and let stand at 4°C for 20 min.
8.Wash the column with 5 ml WGA Gal elution buffer, collecting 0.5-ml fractions.
9.Repeat steps 6-8 using GlcNAc elution buffer.
10.Count 25 μl of each fraction using a liquid scintillation counter.
11.Pool positive fractions that elute in the presence of GlcNAc and precipitate using TCA or methanol.
12.Analyze the pellet by SDS-PAGE and autoradiography (Gallagher, 2001).
Support Protocol 2: DIGESTION OF PROTEINS WITH HEXOSAMINIDASE
Terminal GlcNAc and O-GlcNAc can be removed from proteins using commercially available hexosaminidases; these enzymes will also cleave terminal GalNAc residues. Unlike O-GlcNAcase, commercial hexosaminidases have low pH optima, typically pH 4.0-5.0.
Materials
-
Protein sample for digestion (include a positive control, e.g., ovalbumin)
-
2% (w/v) SDS (see Moore, 2001, for 20% stock)
-
2× hexosaminidase reaction mixture (see recipe)
-
Additional reagents and equipment for SDS-PAGE and electroblotting (Gallagher, 2001)
1.Mix the sample 1:1 with 2% SDS and boil for 5 min.
2.Mix the sample 1:1 with 2× hexosaminidase reaction mixture and incubate at 37°C for 4-24 hr.
3.To assess the completeness of the digestion, separate an aliquot of the reaction by SDS-PAGE and electroblot (Gallagher, 2001) onto an appropriate membrane. Probe blots with carbohydrate-specific lectins or antibodies.
Alternate Protocol: DETECTION OF PROTEINS MODIFIED BY O-GlcNAc USING GALACTOSYLTRANSFERASE
The enzyme β-1,4-galactosyltransferase (from bovine milk) will label any terminal GlcNAc residue with Gal, using uridine diphospho-D-Gal (UDP-Gal) as a donor substrate (Brew, Vanaman, & Hill, 1968). Hart and colleagues have exploited this property, using the enzyme to label terminal GlcNAc residues on proteins with [6-3H]Gal, forming a [3H]βGal1-4βGlcNAc (Greis et al., 1996; Roquemore et al., 1994; Torres & Hart, 1984). The labeled sugar can be chemically released (via β-elimination) and analyzed by size-exclusion chromatography on a BioGel-P4 column, using the 3H radiolabel to detect the fraction of interest (Roquemore et al., 1994). Labeling the O-GlcNAc residue allows the subsequent detection of proteins and peptides of interest during SDS-PAGE, HPLC, protease digestion, and Edman degradation steps. Researchers have been able to identify glycosylation sites on as little as 10 pmol using these methods (Greis et al., 1996). Recently, this technique has been adapted to allow the incorporation of unnatural sugar analogs that can be derivatized to facilitate either the purification or detection of O-GlcNAc-modified proteins and peptides (Kim, 2018), a technique that is marketed by Invitrogen under the “Click-it” brand (Invitrogen).
To achieve efficient labeling of some proteins, it is necessary to denature samples, for example by boiling in the presence of 10 mM DTT and 0.5% (w/v) SDS. Galactosyltransferase has been shown to be active in solutions containing 5 mM DTT, 0.5 M NaCl, up to 2% (v/v) Triton X-100, up to 2% (v/v) NP-40, and 1 M urea. Up to 0.5% (w/v) SDS can be used if it is titrated with a 10-fold molar excess of either Triton X-100 or NP-40 in the final reaction mixture. Digitonin, which is commonly used to solubilize cells, should be used with caution, as it is a substrate for galactosyltransferase. The total ionic strength should be <0.2 M.
Galactosyltransferase requires 1-5 mM Mn2+ for activity but is inhibited by Mg2+ and concentrations of Mn2+ >20 mM. EDTA (or analogs) should be avoided unless titrated with appropriate levels of Mn2+. Note that 1 mole of EDTA binds 2 moles of Mn2+.
Free UDP is also an inhibitor of galactosyltransferase. For studies where complete labeling of the GlcNAc is preferable, such as site mapping, calf intestinal alkaline phosphatase is included in the reaction, as it degrades UDP (Unverzagt et al., 1990). Although this increases the efficiency of the reaction, it is important to add this to the control, as some preparations of alkaline phosphatase contain proteins that will label with galactosyltransferase (R.N. Cole, pers. commun.).
NOTE : Protease inhibitors, such as PIC 1, PIC 2, and PMSF (see the recipe for 1000× protease inhibitors in Reagents and Solutions), can be included (final concentrations, 1×), but GlcNAc and 1-amino-GlcNAc should be removed prior to labeling by spin filtration or another method of desalting.
Materials
-
Protein sample(s)
-
Dithiothreitol (DTT)
-
Sodium dodecyl sulfate (SDS; see Moore, 2001, for 20% stock solution)
-
Label: 1.0 mCi/ml UDP-[3H]Gal (17.6 Ci/mmol; American Radiolabeled Chemicals) in 70% (v/v) ethanol
-
25 mM 5′-adenosine monophosphate (5′-AMP) in Milli-Q water, pH 7.0
-
Buffer H (see recipe)
-
10× galactosyltransferase labeling buffer (see recipe)
-
Galactosyltransferase, autogalactosylated (see Support Protocol 3)
-
Calf intestinal alkaline phosphatase
-
Unlabeled UDP-Gal
-
Stop solution: 10% (w/v) SDS/0.1 M EDTA
-
30 × 1-cm Sephadex G-50 column equilibrated in 50 mM ammonium formate/0.1% (w/v) SDS
-
100°C water bath
-
Speed-Vac evaporator or nitrogen source
-
37°C incubator
-
Liquid scintillation counter
-
Additional reagents and equipment for acetone precipitation of protein (Lovrien & Matulis, 2001), PNGase F digestion of proteins (Powell, 2001), SDS-PAGE (Gallagher, 2001), and product analysis (see Basic Protocol 5)
Prepare the reaction
1.Denature the protein sample by adding DTT to 10 mM and SDS to 0.5% (w/v), and then boiling the sample for 10 min.
2.Decide how many reactions are going to be carried out and thus how much label will be needed (∼1-2 μCi/reaction).
3.Remove solvent from label in a Speed-Vac evaporator or under a stream of nitrogen.
4.Resuspend an appropriate amount of label in 50 μl per reaction of 25 mM 5′-AMP.
5.Set up reactions as follows:
- Up to 50 μl protein sample (final concentration 0.5-5 mg/ml)
- 350 μl buffer H
- 55 μl 10× galactosyltransferase labeling buffer
- 50 μl UDP-[3H]Gal/5′-AMP mixture from step 4
- 30-50 μl autogalactosylated galactosyltransferase
- 1-4 U calf intestinal alkaline phosphatase
- Milli-Q water to a final volume of 550 μl.
6.Incubate 2 hr at 37°C or overnight at 4°C.
7.Add unlabeled UDP-Gal to a final concentration of 0.5-1.0 mM and another 2-5 μl of galactosyltransferase. Incubate for an additional 2 hr at 37°C.
8.Add 50 μl stop solution to each sample and heat to 100°C for 5 min in a water bath.
Isolate the product
9.Resolve the protein from unincorporated label using a Sephadex G-50 column equilibrated in 50 mM ammonium formate/0.1% w/v SDS. Collect 1-ml fractions.
10.Count a 50-μl aliquot of each fraction using a liquid scintillation counter.
11.Combine the void volume and lyophilize to dryness.
12.Resuspend the samples in 1 volume of Milli-Q water (200 ml) and precipitate with 9 volumes of ice-cold acetone (1800 ml; Lovrien & Matulis, 2001).
13.Treat the samples with PNGase F (Powell, 2001), separate by SDS-PAGE, and detect by autoradiography (Gallagher, 2001). Alternatively, subject the samples to “product analysis” to confirm that the label was incorporated onto O-GlcNAc (see Basic Protocol 5).
Support Protocol 3: AUTOGALACTOSYLATION OF GALACTOSYLTRANSFERASE
As galactosyltransferase contains N-linked glycosylation sites, it is necessary to block these before using this enzyme to probe other proteins for terminal GlcNAc.
Materials
-
10× galactosyltransferase labeling buffer (see recipe)
-
Galactosyltransferase (Millipore Sigma, G5507)
-
10,000 U/ml aprotinin
-
2-Mercaptoethanol
-
UDP-Gal
-
Saturated ammonium sulfate: >17.4 g (NH4)2SO4 in 25 ml Milli-Q water, prechilled
-
85% ammonium sulfate: 14 g (NH4)2SO4 in 25 ml Milli-Q water, prechilled
-
Galactosyltransferase storage buffer (see recipe), prechilled on ice
-
30- to 50-ml centrifuge tubes
-
Refrigerated centrifuge
1.Resuspend 25 U galactosyltransferase in 1 ml of 1× galactosyltransferase labeling buffer.
2.Transfer the sample into a 30- to 50-ml centrifuge tube.
3.Remove a 5-μl aliquot for an activity assay.
4.Add 10 μl of 10,000 U/ml aprotinin, 3.5 μl 2-mercaptoethanol, and 1.5-3.0 mg UDP-Gal.
5.Incubate the sample on ice for 30-60 min.
6.Add 5.66 ml of prechilled saturated ammonium sulfate in a dropwise manner over 10 min. Incubate on ice for 30 min.
7.Centrifuge 15 min at >10,000 × g , 4°C, and pour off the supernatant. Resuspend the pellet in 5 ml prechilled 85% ammonium sulfate and incubate on ice for 30 min.
8.Centrifuge 15 min at >10,000 × g , 4°C, and pour off the supernatant.
9.Resuspend the pellet in 1 ml of galactosyltransferase storage buffer and divide into 50-μl aliquots, saving 5 μl for an activity assay as the “auto-gal” sample. Assay that aliquot for activity (see Support Protocol 4).
10.Store the remaining aliquots up to 1 year at −20°C pending use in Alternate Protocol.
Support Protocol 4: ASSAY OF GALACTOSYLTRANSFERASE ACTIVITY
As sample and activity may be lost during the autogalactosylation procedure, it is important to assess the activity of the enzyme.
Materials
-
1.0 mCi/ml UDP-[3H]Gal, (17.6 Ci/mmol; American Radiolabeled Chemicals) in 70% (v/v) ethanol
-
25 mM 5′-adenosine monophosphate (5′-AMP) in Milli-Q water, pH 7.0
-
1× galactosyltransferase dilution buffer: galactosyltransferase storage buffer (see recipe) supplemented with 5 mg/ml BSA
-
“Pre-gal” sample aliquot (see Support Protocol 3, step 3) and “auto-gal” sample aliquot (see Support Protocol 3, step 9)
-
200 mM GlcNAc
-
10× galactosyltransferase labeling buffer (see recipe)
-
Dowex AG1-X8 resin (PO4 form) slurry in 20% (v/v) ethanol
-
Speed-Vac evaporator or nitrogen source
-
37°C incubator
-
Pasteur pipets
-
Glass wool
-
15-ml scintillation vials
-
Liquid scintillation counter
1.Dry 40 μl of 0.1 μCi/μl of UDP-[3H]Gal in a Speed-Vac evaporator or under a stream of nitrogen.
2.Resuspend in 90 μl of 25 mM 5′-AMP.
3.Make 1/1000, 1/10,000, and 1/100,000 serial dilutions of the “pre-gal” and “auto-gal” sample aliquots in 1× galactosyltransferase dilution buffer. Using these dilutions, 200 mM GlcNAc, and 10× galactosyltransferase labeling buffer, prepare reaction mixtures as described in Table 3.
| Sample | Dilution | Vol. (μl) of diluted sample | Vol. (μl) of 200 mM GlcNAc | Vol. (μl) 10× Gal labeling buffer | Vol. (μl) Milli-Q water |
|---|---|---|---|---|---|
| Blank | 0 | 10 | 10 | 70 | |
| Pre-Gal | 1/1000 | 10 | 10 | 10 | 60 |
| 1/10,000 | 10 | 10 | 10 | 60 | |
| 1/100,000 | 10 | 10 | 10 | 60 | |
| Auto-Gal | 1/1000 | 10 | 10 | 10 | 60 |
| 1/10,000 | 10 | 10 | 10 | 60 | |
| 1/100,000 | 10 | 10 | 10 | 60 |
4.Start the reaction by adding 10 μl of 0.05 μCi/μl UDP-[3H]Gal (see step 2) to each tube.
5.Incubate the samples 30 min at 37°C.
6.Pour 1 ml of Dowex AG1-X8 slurry (PO4 form) into 13 Pasteur pipets, each plugged with a small amount of glass wool.
7.Wash with at least 3 ml Milli-Q water. Do not let the columns run dry.
8.When almost all the Milli-Q water has eluted, place each column over a separate 15-ml scintillation vial.
9.Stop the reaction (still incubating from step 5) by adding 500 μl Milli-Q water.
10.Load each sample onto the corresponding column and add a 500 μl water wash of the tube. Collect eluate as fraction A.
11.Elute with two 1-ml additions of Milli-Q water. Collect eluates as fractions B and C, respectively.
12.Count 100 μl of the sample (fractions A, B, and C) using a liquid scintillation counter. The activity can be expressed either as dpm 3H incorporation onto the GlcNAc residue or as μmol 3H incorporation (1 μCi = 2.22 × 106 dpm).
Basic Protocol 5: CHARACTERIZATION OF LABELED GLYCANS BY β-ELIMINATION AND CHROMATOGRAPHY
This protocol has three steps: (1) the release of carbohydrates as sugar alditols by reductive β-elimination; (2) desalting of the sample, with concomitant confirmation of the size of the labeled sugar alditol(s); and (3) confirmation that the product is [3H]βGal1-4βGlcNAcitol (from galactosyltransferase labeling).
Materials
-
Radiolabeled glycoproteins (Alternate Protocol)
-
β-elimination reagent: 1 M NaBH4/0.1 M NaOH (prepare fresh)
-
4 M acetic acid, prechilled on ice
-
1.5-ml screw-cap microcentrifuge tubes
-
37°C incubator
-
Ice
-
Additional reagents and equipment for acetone or methanol precipitation of proteins (Lovrien & Matulis, 2001), size-exclusion (gel-filtration) chromatography (Boysen & Hearn, 2001), and Dionex chromatography (Townsend, Basa, & Spellman, 1996; Townsend & Hardy, 1991; Townsend, Hardy, Cumming, Carver, & Bendiak, 1989)
1.Acetone- or methanol-precipitate the labeled proteins (Lovrien & Matulis, 2001) in 1.5-ml screw-cap microcentrifuge tubes.
2.Resuspend the sample in 500 μl β-elimination reagent and incubate 18 hr at 37°C.
3.Cool the sample on ice.
4.Neutralize the reaction by adding 5 μl ice-cold 4 M acetic acid in a stepwise manner. Check that the pH is between pH 6 and 7.
5.Resuspend the sugar alditols in Milli-Q water. Analyze by size-exclusion chromatography (Boysen & Hearn, 2001) or by Dionex chromatography (Townsend et al., 1989, 1996; Townsend & Hardy, 1991).
Basic Protocol 6: DETECTION OF O-GlcNAc IN 96-WELL PLATES
The ability to detect O-GlcNAc in a 96-well plate format allows the rapid optimization of treatments that alter protein O-GlcNAcylation levels in vivo. The method described below, a quantitative immunofluorescence assay, can be used either directly in a 96-well plate reader or in cell imaging platforms. This protocol was optimized using three separate cell types (U2OS, mouse embryonic fibroblasts, and Cos-7); however, it is ideal to ensure that the O-GlcNAc levels of new cell types are within the linear range before assessing treatments that alter O-GlcNAc levels.
Materials
-
Cells of interest and appropriate culture medium
-
PBS, pH 7.4 (Moore, 2001)
-
MES buffer: 100 mM MES/1 mM EDTA/1 mM MgCl2 pH 6.9 (store at 4°C)
-
Fixation solution 1 (1:10 [v/v] MES buffer/methanol; store at −20°C) or fixation solution 2 (4% [v/v] paraformaldehyde in PBS; store at 4°C)
-
Permeabilization solution: 0.5% (v/v) Triton-X-100 in PBS
-
TBST (see recipe)
-
Blocking buffer: 3% (w/v) BSA in TBST
-
Nuclear counterstain: SYTO 62 (5 mM) Red Fluorescent Nucleic Acid Stain (Thermo Fisher Scientific, S11344), diluted 1:2000-1:10,000 (to 2.5-0.5 µM) in blocking buffer
-
Competitive blocking buffer: Blocking buffer containing 500 mM GlcNAc
-
Primary antibody dilution:
- CTD110.6 (Millipore Sigma, MABS1254): 2 μg/ml in blocking buffer; or
- RL2 (Millipore Sigma, MABS157): 2 μg/ml in blocking buffer
-
Optional : Actin counterstain: Alexa Fluor 647 Phalloidin (Thermo Fisher Scientific, A22287; use only with paraformaldehyde-fixed cells, diluted in blocking buffer to the manufacturer's recommended working concentration)
-
Fluorescent secondary antibody dilutions: Anti-mouse IgM Alexa Fluor 488 (Thermo Fisher Scientific, A-21042) and anti-rabbit IgG Alexa Fluor 488 (Thermo Fisher Scientific, A-32731/Bethyl Laboratories A120-201D2), diluted to 2-4 µg/ml in blocking buffer
-
Cell culture hood
-
Incubator
-
96-well, black-wall, clear-bottom plates (Corning, 3603)
-
Aspirator
-
Glass Pasteur pipets
-
10-μl plastic pipet tips
-
Vortex with shaking attachment (or alternative)
-
Plate reader such as Spectramax plate reader (Molecular Devices)
-
Optional : Minimax 300 cell imager system
-
Aluminum foil or plate covers
-
Ice
Prepare and plate the cells
1.Split cells and resuspend to a concentration of 5 × 104 cells/ml.
2.Plate 100 μl in 96-well, black-wall, clear-bottom plates, including wells with medium alone (blanks).
3.Allow cells to grow to 70%-90% confluence.
4.Perform experimental manipulations as necessary, such as treatment with an inhibitor that would alter protein O-GlcNAcylation (see Basic Protocol 1).
Cell fixation and staining
5.Wash away the medium:
-
Chill PBS to 4°C.
-
Remove medium from the microwell plate by aspiration.
Be sure to use a 10-μl plastic pipet tip to cover the end of the glass Pasteur pipet. This helps prevent the loss of cells during aspiration.
Inverting the plate to remove the medium and blotting the open face of the plate also reduces cell loss.
- Wash by adding 100 μl PBS, letting stand for 3 min, and aspirating off the buffer.
Add the solution carefully by pipetting down the side of the wells to avoid detaching the cells.
6.Fix the cells:
- Immediately fix cells by addition of 100 μl of fixation solution 1 or 2 (MES/methanol or 4% (v/v) paraformaldehyde) and incubate the microwell plate on ice for 15 min.
Fixation solution 1 (MES/methanol) rapidly dehydrates and precipitates protein while maintaining secondary structure. As methanol does not cross-link proteins, there is less likelihood that protein epitopes will be altered so as to reduce antibody binding. Methanol also has the added effect of permeabilizing the cell membrane, which leads to loss of some cytoplasmic contents, particularly small molecules and lipophilic species.
The paraformaldehyde in fixation buffer 2 cross-links proteins and nucleic acids with good preservation of the cellular spatial information and composition. Prolonged fixation increases the risk of epitope alteration and reduced antibody binding. Methanol-free paraformaldehyde is the recommended fixative for phalloidin stains.
-
Remove the fixation solution by aspiration.
-
Wash the cells with 100 μl PBS three times for 3 min each.
-
Proceed to permeabilization (step 7).
If the cells are not processed with primary antibodies on the same day, you can store the fixed cells in 100 μl PBS at 4°C (≤96 hr) until ready for immunostaining. When ready to process the cells, aspirate the storage buffer and proceed with permeabilization.
7.Permeabilize the cells:
- Add 100 μl permeabilization buffer and incubate 10 min at room temperature.
IMPORTANT NOTE: Do not shake the plate.
-
Aspirate buffer.
-
Add 100 μl TBST to each well and incubate 3 min at room temperature. Repeat two more times.
8.Block the cells: Carefully add 150 μl blocking buffer down the sides of the wells and incubate 1.5 hr at room temperature with mild rocking.
9.Wash the cells:
-
Remove the blocking buffer by aspiration.
-
Add 200 μl TBST to the wells, incubate 3 min, and aspirate. Repeat two more times.
10.Counterstain the cells:
- Prepare the nuclear or actin fluorescent counterstain as necessary.
Some cells do not contain nuclei (red blood cells), whereas others can be multinuclear. In this case, actin is a more appropriate counterstain.
-
Add 100 µl of the diluted counterstain to the cells and incubate 30 min at room temperature.
-
Aspirate the stain.
-
Add 200 μl TBST to the well, incubate 3 min, and aspirate. Repeat two more times.
OPTIONAL: The nuclear counterstaining can be performed simultaneously with the blocking step by making the stain dilution in the blocking buffer.
11.Add primary antibodies:
-
Prepare O-GlcNAc antibody dilutions in blocking buffer and competitive blocking buffer as necessary.
-
Add 100 μl of the desired primary antibody combinations to the desired wells. The antibody solution should cover the bottom of each well.
-
Incubate overnight at 4°C with gentle shaking. Seal the plate around the edges with parafilm to reduce evaporation.
If you are using a primary antibody conjugated to a fluorophore, you should also cover the plate with foil to avoid photobleaching of the dye.
12.Wash the cells:
-
Add 200 μl TBST by gently adding buffer down the sides of the wells to avoid detaching the cells, incubate 3 min, and aspirate. Repeat two more times.
-
Optional: Rock the plate gently during the wash steps.
13.Add the secondary antibody:
- Dilute the fluorescent-dye-labeled secondary antibodies in blocking buffer.
To lower nonspecific binding, add Tween 20 to the diluted antibody to a final concentration of 0.2% (v/v).
- Add 100 μl of the secondary antibody solution to each well and incubate 60 min at room temperature.
Protect the plate from light during incubation.
IMPORTANT NOTE: Some of the secondary antibodies are at different concentrations (in mg/mL). When choosing different secondary conjugates, it may be useful to try multiple dilutions or adjust the μg/mL concentration to match a previously validated dilution.
14.Wash the cells:
-
Wash the plate with TBST by gently adding 200 μl of buffer down the sides of the wells to avoid detaching the cells.
-
Incubate for 3 min at room temperature and aspirate the buffer. Repeat two more times.
-
After the final wash, turn the plate upside down and blot gently on paper towels to remove traces of wash buffer.
-
Add 100 μl PBS per well for storage or imaging.
For best results, scan plate immediately; plates may also be stored at 4°C in commercial mounting medium with antifade (protected from light).
-
Seal the plate in parafilm to prevent evaporation, and protect plates from light until imaging to ensure the highest sensitivity.
15.Scan the plate:
-
Before scanning, clean the bottom plate surface with a damp Kimwipe (with water, not ethanol) to remove stains.
-
Scan the plate using cell imaging functionality for fluorescence and transmitted light. Alternatively, scan the plate using the conventional fluorescence plate reader functionality.
-
When storing plates after imaging, they should be sealed and remain protected from light at 4°C.
-
The O-GlcNAc signal can be calculated by deducting signals from the equivalent competition well.
Well-to-well variability arising from variations in cell number can be addressed by rescaling the O-GlcNAc signal to the average counterstain intensity (DNA or actin) for that well.
When optimizing treatments, a signal-to-noise ratio of at least 3 is desirable.
Basic Protocol 7: ASSAY FOR OGT ACTIVITY
The detection and analysis of O-GlcNAc on proteins is only the first step in the analysis of O-GlcNAc and the protein(s) of interest. More important is determining the function of the modification. Protocols for the analysis of the enzymes that add and remove O-GlcNAc have been included in this unit, as they may aid in understanding the role of O-GlcNAc in a particular model. Recent examples where studies such as this have been critical include those that have shown the reciprocity between O-GlcNAc and O-phosphate on the C-terminal domain of RNA Pol II (Comer & Hart, 2001); studies showing elevated activity of enzymes in certain tissue/cell lines and tissue fractions (Whelan, Lane, & Hart, 2008); and, finally, studies indicating that the enzymes responsible for the addition and removal of O-GlcNAc copurify with kinases and phosphatases (Wells, Kreppel, Comer, Wadzinski, & Hart, 2004).
O-GlcNAc transferase (OGT), or uridine diphospho-N -acetylglucosamine:polypeptide β-N -acetylglucosaminyltransferase, transfers GlcNAc to the hydroxyl groups of Ser and Thr residues of proteins and peptides using UDP-GlcNAc as a donor substrate (Haltiwanger, Blomberg, & Hart, 1992; Haltiwanger, Holt, & Hart, 1990; Kreppel, Blomberg, & Hart, 1997). OGT activity is assayed by determining the rate at which [3H]GlcNAc is transferred to an acceptor peptide. A number of peptides have been identified as substrates for OGT in vitro, but a peptide (340PGGSTPVSSANMM352) from the α-subunit of casein kinase II (CKII) is an efficient in vitro substrate that is commonly used (Kreppel & Hart, 1999).
OGT activity can be assayed in crude preparations (Haltiwanger et al., 1990) or using recombinant protein (Kreppel & Hart, 1999). OGT activity is sensitive to salt inhibition and reducing agents, so it is important to desalt the preparation before assaying if high salt concentrations are present (see Support Protocol 5). Typically, 0.2-1 μg of purified recombinant protein is used per assay. To assay OGT activity in cell/tissue lysate, 10-50 μg of desalted lysate is required per assay.
Materials
-
0.1 mCi/ml UDP-[3H]GlcNAc (20-45 Ci/mmol; American Radiolabeled Chemicals) in 70% (v/v) ethanol
-
25 mM 5′-adenosine monophosphate (5′-AMP), in Milli-Q water, pH 7.0
-
Crude or purified OGT sample, desalted (see Support Protocol 5)
-
10× OGT assay buffer (see recipe)
-
CKII peptide substrate (OGT peptide): +H2N-PGGSTPVSSANMM-COO− (custom synthesized and dissolved in H2O to 10 mM, with pH adjusted to 7, if necessary)
-
0.5 mM Thiamet-G in 0.5 M HEPES, pH 7.5
-
20 U/μl calf intestinal alkaline phosphatase (18009019; Thermo Fisher Scientific)
-
Quenching buffer: 50 mM formic acid/500 mM NaCl
-
50 mM formic acid
-
Acetone
-
Methanol (HPLC-grade)
-
Scintillation fluid
-
Speed-Vac evaporator or nitrogen source
-
Clear round-bottom 96-well plate
-
Strata C18 96-well plate (25 mg/well; 8E-S001-CGB; Phenomenex)
-
Scintillation counter
1.Dry down an aliquot of UDP-[3H]GlcNAc in a Speed-Vac evaporator or under a stream of nitrogen just before use. Resuspend in an appropriate volume of 25 mM 5′-AMP, so that the concentration is 1 μCi/μl.
2.Prepare a master mix containing each of the following per reaction:
- 5 μl of 10× OGT assay buffer
- 5 μl of 10 mM CKII peptide substrate
- 0.5 μl 1 μCi/μl UDP-[3H]GlcNAc (0.5 μCi per reaction)
- 1 μl of 0.5 mM Thiamet-G
- 0.25 μl of calf intestinal alkaline phosphatase (5 U per reaction)
- H2O to 20 μl.
3.Array master mix in a clear round-bottom 96-well plate and initiate reactions by adding 30 μl of desalted OGT.
4.Incubate for 30 min to 1 hr at room temperature.
5.Stop the reaction by adding 150 μl quenching buffer.
6.Activate a C18 96-well plate (Phenomonex; 25 mg resin/well) with 100% acetone (1.5 ml). Once the acetone has passed through the columns, add methanol (1.5 ml). Finally, equilibrate the columns by adding 1.5 ml quenching buffer; repeat two more times.
7.Load the reaction (200 μl total) onto the 96-well plate. Wash the plate sequentially with quenching buffer, water, and 50 mM formic acid (two times with 1.5 ml each).
8.Elute the peptide with 1.5 ml of 100% methanol.
9.Add 0.75 ml of the eluate to 5 ml scintillation fluid and count 3H. Calculate OGT activity as peptide micromoles of GlcNAc incorporated, per minute per milligram of protein.
Support Protocol 5: DESALTING OF O-GlcNAc TRANSFERASE
OGT activity is sensitive to salt inhibition (IC50 = 40-50 mM NaCl). It is important to desalt the enzyme preparation before setting up the assay if a high concentration of salt is present. Additionally, O-GlcNAcase activity is sensitive to detergent present in extraction buffers. The protocol described below is also used to desalt O-GlcNAcase before setting up assay reactions.
Materials
-
OGT desalting buffer (see recipe)
-
Sample: volume range 20-100 μl
-
Zeba 96-well Spin Desalting Plates, 40K MWCO (87774; Thermo Fisher Scientific)
-
96-well wash plates
-
96-well collection plates
-
Ice
1.Place the desalting plate on top of the wash plate and centrifuge 2 min at 1000 × g , 4°C, to remove the storage solution.
2.Discard the flowthrough and replace the desalting plate onto the wash plate.
3.Add 250 μl desalting buffer on top of the resin. Centrifuge and discard flowthrough. Repeat this step two additional times.
4.Blot the bottom of the desalting plate to remove excess liquid and place it on top of a new collection plate.
5.Load the protein sample (20-100 μl) onto the resin. Centrifuge 3 min at 1000 × g , 4°C, and retain the flowthrough that contains the sample.
Basic Protocol 8: ASSAY FOR O-GlcNAcase ACTIVITY
O-GlcNAcase, also known as N -acetylglucosaminidase or hexosaminidase C (EC 3.2.1.52), is a cytosolic glycosidase specific for O-linked β-GlcNAc. The activity of O-GlcNAcase can be conveniently assayed in vitro with a fluorogenic substrate, 4-methylumbelliferyl-GlcNAc (4MU-β-GlcNAc). The cleavage product, 4-methylumbelliferone (4MU), is fluorescent with an emission at 460 nm (Macauley et al., 2005).
Materials
-
Purified O-GlcNAcase (0.2-1 μg) or cell/tissue extract (10-50 μg, desalted; see annotation following step 1)
-
10× O-GlcNAcase assay buffer (see recipe)
-
1 M GalNAc
-
100 mM 4MU-βGlcNAc in dimethyl sulfoxide (DMSO; Millipore Sigma, M2133)
-
100 mM 4MU-βGalNAc in DMSO (Millipore Sigma, M9659)
-
OGT desalting buffer (see recipe)
-
Free 4MU standards: 100 mM 4MU in DMSO
-
Positive control: 5000 U/ml β-N -acetylhexosaminidasef (New England Biolabs, P0721)
-
Quenching buffer: 200 mM glycine, pH 10.75
-
Black flat-bottomed 96-well plate and sealing tape
-
Centrifuge, 4°C
-
37°C incubator
-
Plate reader
1.Prepare O-GlcNAcase.
2.Precool a 96-well plate or microcentrifuge tubes on ice.
3.Set up reactions in triplicate in the precooled plate wells or tubes by adding 20 μl partially purified O-GlcNAcase enzyme or cell/tissue extract to each well or tube as appropriate.
4.Prepare a master mix containing each of the following per sample:
- 5 μl 10× O-GlcNAcase assay buffer
- 5 μl 1 M GalNAc
- 0.5 μl 4MU-GlcNAc
- 19.5 μl H2O.
50 mM GalNAc (final) is included in the reaction to inhibit lysosomal hexosaminidases A and B that may be present in the enzyme preparation. O-GlcNAcase is not inhibited by 50 mM GalNAc.
- Prepare a similar master mix containing 4MU-GalNAc instead of 4MU-GlcNAc as a substrate.
4MU-GalNAc acts as a negative control substrate that allows an assessment of the activity of any contaminating lysosomal hexosaminidases.
5.Add 30 µl of the appropriate master mix to each sample.
6.Prepare a 4MU standard curve (0-10 nmol) in DMSO. Add 50 μl of each dilution to separate wells in triplicate.
7.Quench one reaction of the three from every triplicate reaction set up at time point zero by adding 150 μl quenching buffer (200 mM glycine, pH 10.75).
8.Mix well and cover.
9.Incubate 30-60 min at 37°C.
10.At the end of the incubation, add 150 μl quenching buffer (200 mM glycine, pH 10.75) to the remaining reactions, including the 4MU standards.
11.Read the fluorescence intensity with an excitation wavelength at 368 nm and an emission wavelength of 450 nm on a plate reader.
12.Calculate O-GlcNAcase activity according to the following equation:
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see Moore (2001).
Biosynthetic labeling medium
Glucose-free culture medium containing :
- 50 μCi/ml D-[6-3H]glucosamine (22 Ci/mmol; American Radiolabeled Chemicals, ART 0110A)
- 10% (v/v) FBS
- Prepare fresh
Buffer H
- 50 mM HEPES, pH 6.8
- 50 mM NaCl
- 2% (v/v) Triton X-100
- Store up to 1 month at room temperature.
Citrate-phosphate buffer, pH 4.0, 2×
Dissolve 12.9 g citric acid monohydrate (mol. wt. 210) and 20.6 g disodium hydrogen phosphate heptahydrate (Na2HPO4·6H2O) in 300 ml Milli-Q water. Bring volume to 500 ml. Divide into 10-ml aliquots and store up to 1 year at −20°C.
Galactosyltransferase labeling buffer, 10×
- 100 mM HEPES, pH 7.5
- 100 mM galactose
- 50 mM MnCl2
- Store up to 1 month at 4°C.
Galactosyltransferase storage buffer
- 2.5 mM HEPES, pH 7.4
- 2.5 mM MnCl2
- 50% (v/v) glycerol
- Store up to 1 month at room temperature.
Hexosaminidase reaction mixture, 2×
Per reaction :
- 25 μl 2× citrate-phosphate buffer (see recipe)
- 1 U N -acetyl-β-D-glucosaminidase (V-Labs)
- 0.01 U aprotinin
- 1 μg leupeptin
- 1 μg α2-macroglobulin
- Prepare fresh
O-GlcNAcase assay buffer, 10×
- 500 mM sodium cacodylate, pH 6.4
- 3% (w/v) bovine serum albumin (BSA)
- Prepare fresh
OGT assay buffer, 10×
- 500 mM sodium cacodylate, pH 6.0
- 10 mg/ml bovine serum albumin (BSA)
- Prepare fresh
OGT desalting buffer
- 20 mM Tris·Cl, pH 7.8 (Moore, 2001)
- 1 mg/ml bovine serum albumin (BSA)
- 20% (v/v) glycerol
- 0.02% (w/v) NaN3
- Store up to 1 week at 4°C.
Protease inhibitors, 1000×
PIC 1, 1000× :
- Dissolve the following in 10,000 U/ml aprotinin solution (Millipore SIGMA)
- 1 mg/ml leupeptin
- 2 mg/ml antipain
- 10 mg/ml benzamide
PIC 2, 1000× :
- Prepare in DMSO
- 1 mg/ml chemostatin
- 2 mg/ml pepstatin
PMSF, 1000× :
- 0.1 M phenylmethylsulfonyl fluoride in 95% ethanol
TBST
- 10 mM Tris·Cl, pH 7.5 (Moore, 2001)
- 150 mM NaCl
- 0.05% (v/v) Tween 20
- Store up to 1 month at room temperature.
Tris-buffered saline (TBS)
- 10 mM Tris·Cl, pH 7.5 (Moore, 2001)
- 150 mM NaCl
- Store up to 1 month at room temperature.
WGA Gal elution buffer
Phosphate-buffered saline (PBS; Moore , 2001 ) containing :
- 0.2% (v/v) NP-40
- 1 M D-(+)-galactose (Gal)
- Store up to 1 week at 4°C.
WGA GlcNAc elution buffer
Phosphate-buffered saline (PBS; Moore , 2001 ) containing :
- 0.2% (v/v) NP-40
- 1 M N -acetylglucosamine (GlcNAc)
- Store up to 1 week at 4°C.
COMMENTARY
Background Information
(β)-D-1,4-Galactosylaminyltransferase from bovine milk recognizes terminal N -acetylglucosamine (GlcNAc) residues and modifies them by the addition of a single Gal residue. Torres and Hart first used this enzyme in combination with UDP-[3H]Gal to demonstrate that bovine lymphocytes contain proteins modified by O-linked GlcNAc (Torres & Hart, 1984). Further refinements of this experiment led them to propose that the product, βGal1-4βGlcNAc, was the result of the galactosyltransferase recognizing and modifying a single GlcNAc residue O-linked to Ser/Thr residues of nuclear and cytoplasmic proteins (Holt & Hart, 1986). Since this report, many cytosolic and nuclear proteins from mammalian cells have been shown to be modified by O-GlcNAc. This method has remained the “gold standard” technique to detect O-GlcNAc-modified proteins, as the label provides a “tag” for subsequent analyses, such as those described under “Product characterization” in Critical Parameters and Troubleshooting, below. This approach has been modified to include sugars with bioorthogonal handles that can be further modified by probes allowing the detection and enrichment of glycans. Reagents for this approach, colloquially referred to as “click” chemistry, are marketed by Invitrogen (as reviewed in depth by Kim, 2018). As described in Basic Protocol 1, methods such as WGA affinity and immunoblotting with GlcNAc-specific lectins and antibodies have become popular as simple techniques for the initial characterization of target proteins.
Critical Parameters and Troubleshooting
Extraction of proteins from cells
The O-GlcNAc modification can be removed from proteins by either cytosolic O-GlcNAcase or lysosomal hexosaminidases. The inclusion of inhibitors during the extraction and purification process will preserve the levels of O-GlcNAc on proteins. Commonly used inhibitors (Dong & Hart, 1994) include 1-amino-GlcNAc (1 mM), GlcNAc (100 mM), PUGNAc (5 μM), and Thiamet G (1 μM). Note that these may have to be removed, as they will act as inhibitors in other methods.
Product characterization
Product characterization is a critical step to show that a protein is modified by O-GlcNAc and not other glycans. Although many proteins modified by O-GlcNAc have been identified, evidence from metabolic labeling (Medina, Grove, & Haltiwanger, 1998) and lectin labeling studies (Hart, Haltiwanger, Holt, & Kelly, 1989) suggests that O-GlcNAc is not the only intracellular carbohydrate post-translational modification. In addition, at least one peptide mimic of O-GlcNAc has been identified in cytokeratins (Shikhman, Greenspan, & Cunningham, 1993, 1994). Moreover, many techniques used for breaking open cells also release proteins that are modified by complex N- and O-linked sugars, which may contain terminal GlcNAc. Many of the techniques described in this article will recognize any terminal βGlcNAc residue, and it is important to perform the described controls, such as PNGase F digestion, to demonstrate specificity.
Product analysis is critical for metabolic labeling with glucosamine. Although UDP-GlcNAc is the major product, glucosamine can enter other biosynthetic pathways, such as those used for amino acid synthesis. This issue was highlighted by studies of the SV40 large T antigen. Some researchers have found that the SV40 large T-antigen labels with a number of different tritiated carbohydrates. However, O-GlcNAc is the only carbohydrate post-translational modification of the SV40 large T antigen. The incorporation of glucosamine into amino acid biosynthetic pathways could be reduced by growing cells in the presence of excess nonessential amino acids (Medina et al., 1998).
Lastly, although galactosyltransferase is specific for terminal GlcNAc residues, researchers (Elling et al., 1999) have shown that galactosyltransferase will modify GlcNAc linked in either the α- or β-anomeric conformation. The authors of this protocol have shown that proteins modified by α-O-GlcNAc will be labeled using the procedure described (N. Zachara, unpub. observ.). Although α-O-GlcNAc has not been identified in complex eukaryotes, it is a common modification of cell surface proteins of simple eukaryotes, such as trypanosomes and Dictyostelium. Product analysis, such as HPAEC of the sugar alditols, will resolve many of the issues discussed.
Understanding Results
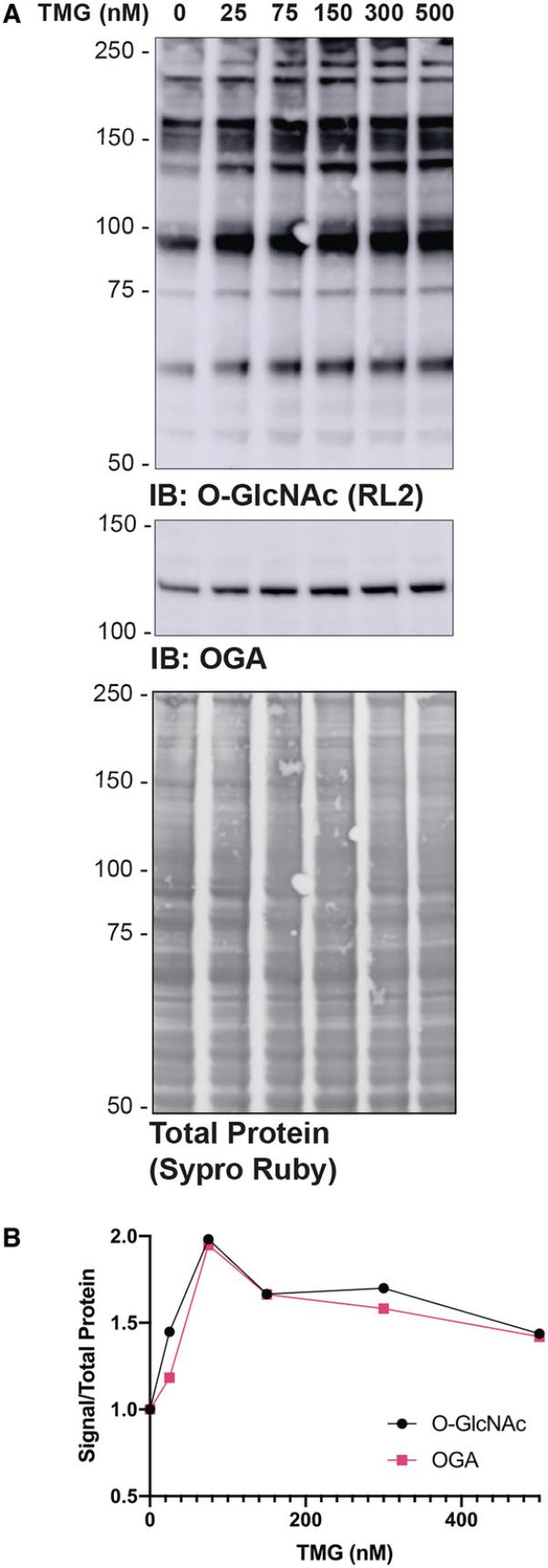
Modulating the abundance of O-GlcNAc is a useful control for detection methodologies, as well as for testing the impact of O-GlcNAc on protein or pathway function. It is ideal to choose the shortest treatment with the lowest dose, as these treatments are less likely to have pleiotropic effects. To optimize treatments (Basic Protocol 1), inhibitor dose or application time can be varied. Figure 1 presents data in which the dose of inhibitor (Thiamet-G) has been modulated over 4 hr. In that example, there is a linear relationship between O-GlcNAc and inhibitor dose from 75 nM. As previously reported, elevating O-GlcNAc levels also augments the abundance of OGA (Kazemi, Chang, Haserodt, McKen, & Zachara, 2010; Park et al., 2017; Tan et al., 2020).
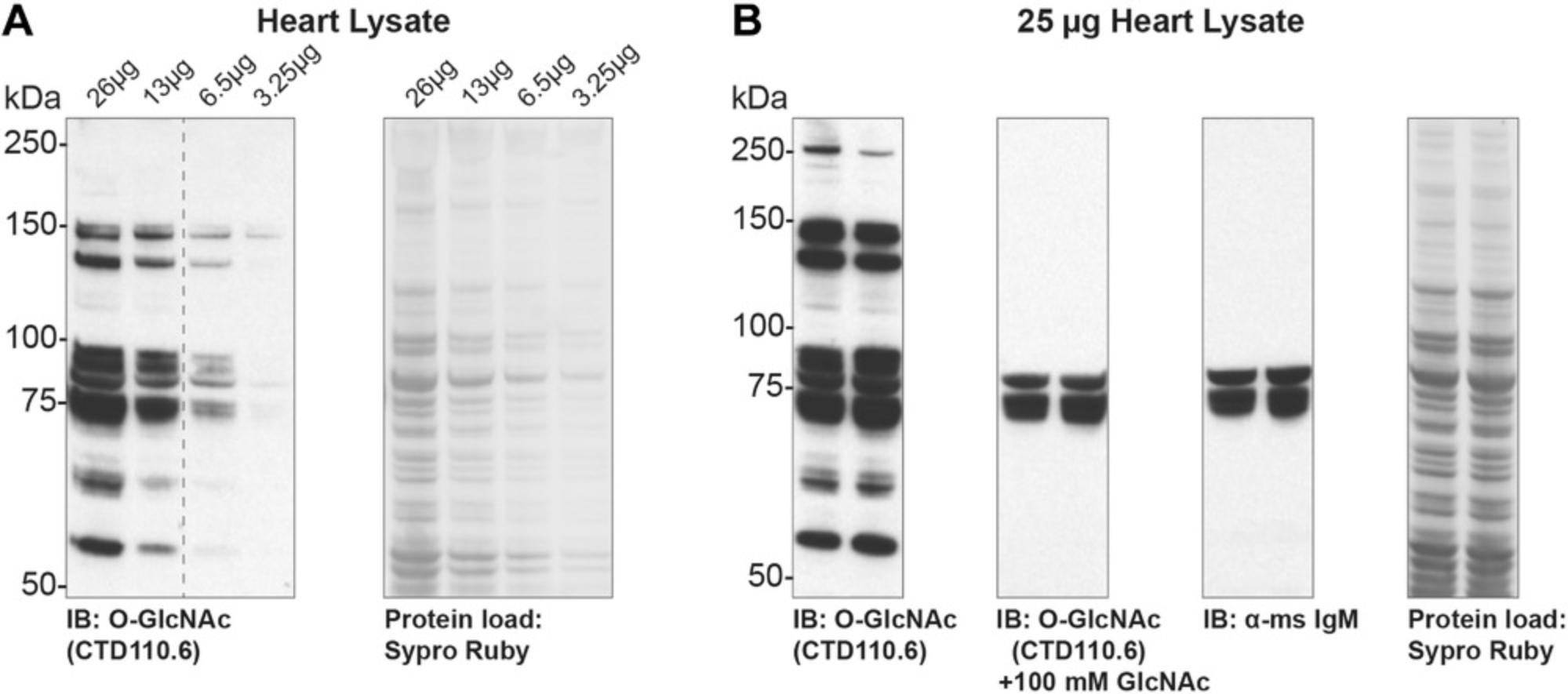
When detecting O-GlcNAc by immunoblotting or lectin blotting (Basic Protocol 2, Basic Protocol 3), it is critical to assess both linear range and specificity. Linear range will differ based on cell and tissue type, as well as the detection modality (ECL, fluorescence, reagents, film) and source of reagents (antibody concentration). Linear range is addressed simply by loading a dilution series of a protein of interest, detecting O-GlcNAc, and quantifying the O-GlcNAc and protein signals (Fig. 2A). In this example, quantitation reveals that the signal is linear from 13 to 26 mg of protein. Specificity can be assessed by modulating O-GlcNAc levels (Basic Protocol 1, Support Protocol 1, and Support Protocol 2) and competing away primary antibody with free GlcNAc. This latter control is critical in immunoprecipitations and tissues, especially mouse tissues, in which immunoglobulin is present (Fig. 2B). In the example shown (heart tissue), use of free GlcNAc or secondary antibody alone highlights two immune-reactive bands at ∼70 kDa that represent mouse IgM heavy chain. A similar band at 55 kDa is observed when probing with RL2, a mouse IgG.
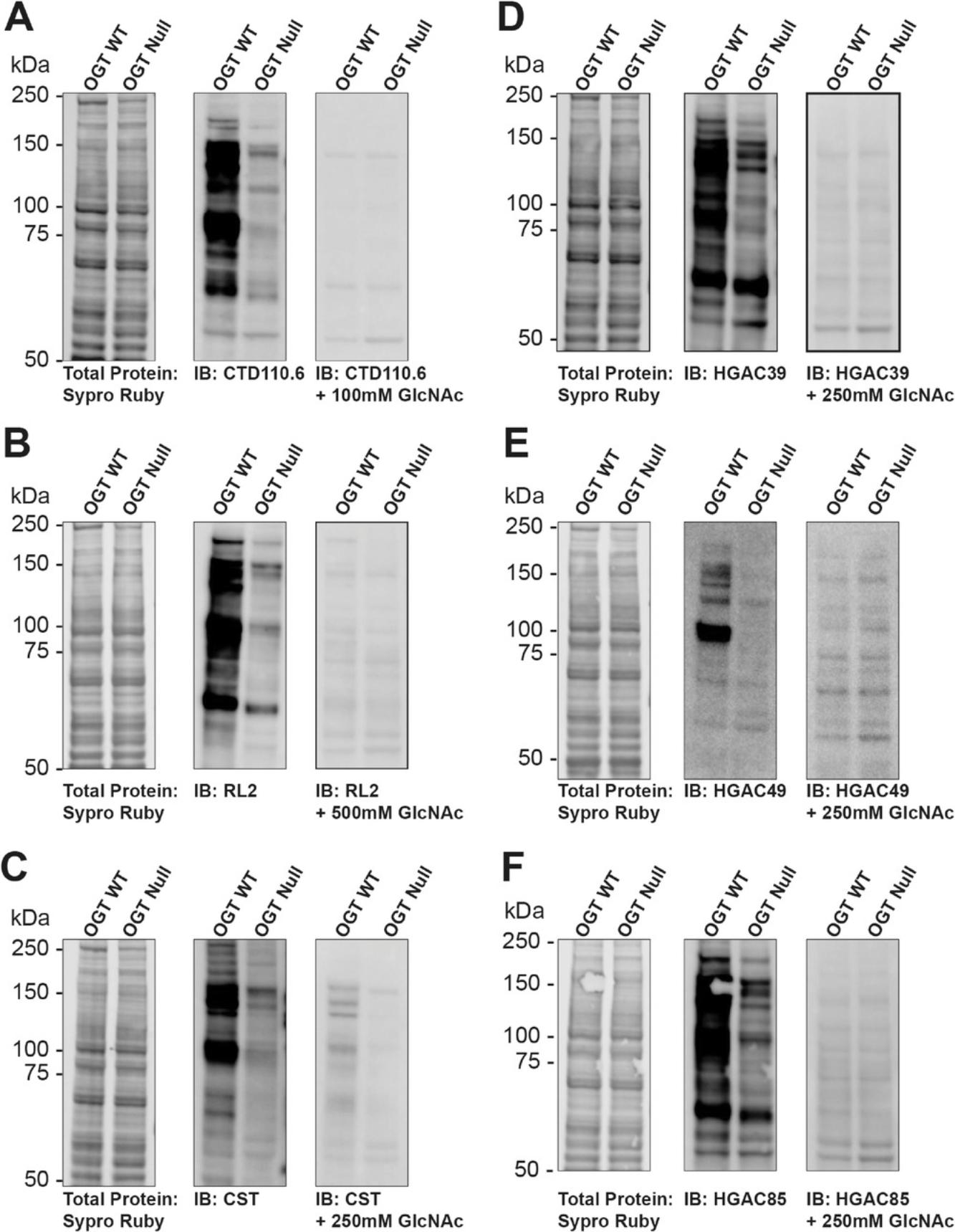
There are numerous antibodies that can be used to detect the O-GlcNAc modification. Figure 3 presents representative data for six O-GlcNAc pan-specific antibodies. In addition to modulating O-GlcNAc levels (OGT WT and OGT null), the signal has been competed away with free GlcNAc. Confirmation of specific O-GlcNAc signals can be achieved by free GlcNAc competition and/or signal reduction with deletion of OGT. As previously reported, each of these antibodies displays different preferences for substrates, and this is represented by the different binding patterns (Lee et al., 2016; Reeves et al., 2014; Snow et al., 1987; Teo et al., 2010). Analogous data and controls should be obtained when using sWGA (Basic Protocol 3). Screening of antibodies and lectins in different models is warranted as some proteins, such as α-synuclein, are not well recognized by antibodies (Levine et al., 2019). For such proteins, the use of galactosyltransferase or metabolic labeling is necessary.
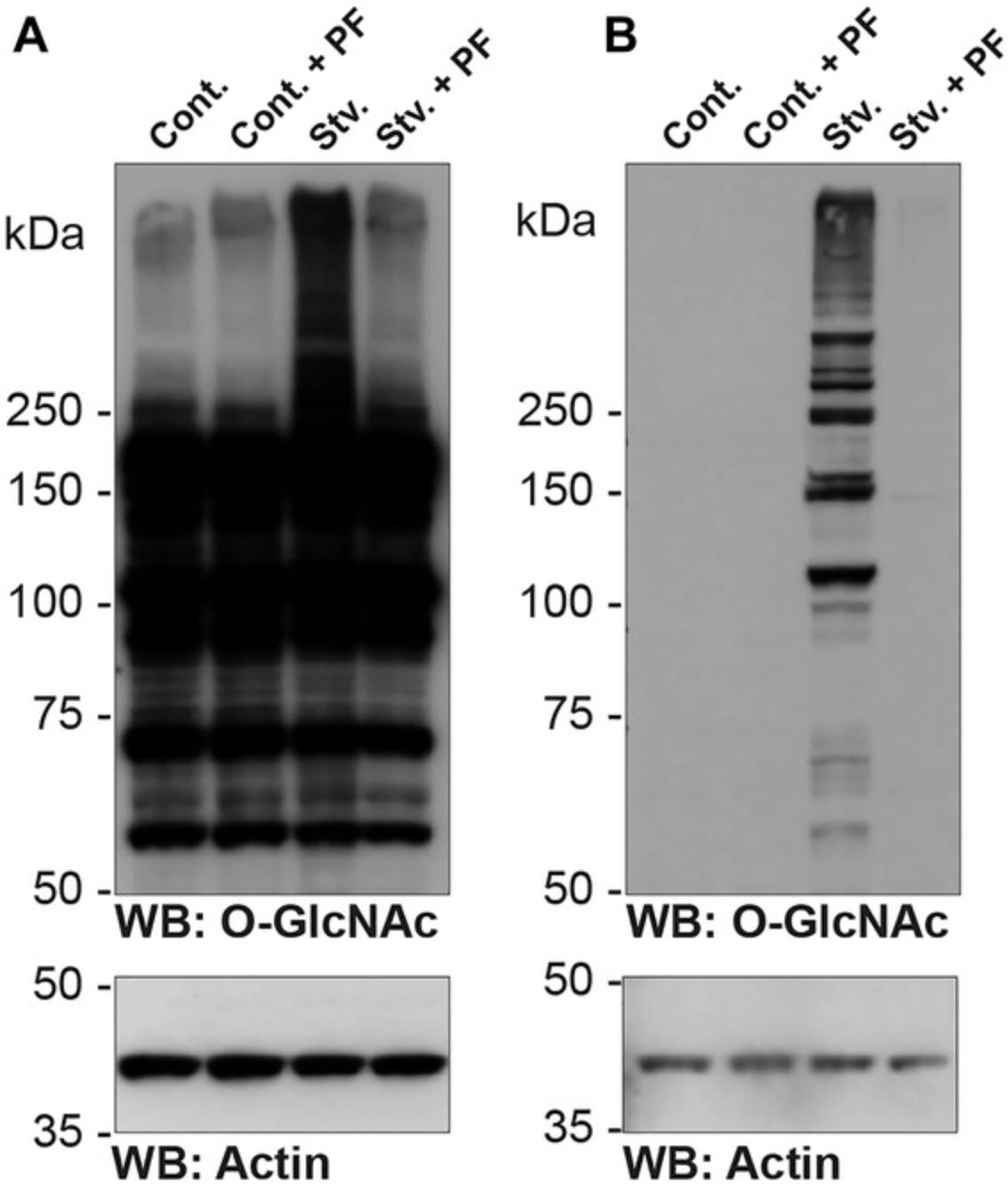
A subset of pan-O-GlcNAc reactive antibodies cross-react with abbreviated N-linked glycans, as well as bisecting GlcNAc residues on N-linked glycans (Isono, 2011; Reeves et al., 2014; Tashima & Stanley, 2014). Although the occurrence of the abbreviated N-linked glycan appears restricted to mammalian cells/tissues exposed to nutrient deprivation and protozoa, the ability to differentiate between an N- and an O-linked GlcNAc residue is critical. Support Protocol 1 presents such a method. In the example shown, cells have been subjected to nutrient deprivation (STV: 0 mM glucose, 1% FBS; 24 hr), which results in a robust increase in CTD110.6 signal that is sensitive to PNGase F (Fig. 4A, lane 3). In control cells, the majority of the signal is sensitive to β-elimination, suggesting that it arises from O-GlcNAc (Fig. 4B). In contrast, a portion of the CTD110.6 signal in starved cells is resistant to β-elimination (Fig. 4B). Sodium hydroxide will destroy protein epitopes. Thus, although a modest decrease in protein signals is expected (compare actin signals in Fig. 4A and B), the signal should not be destroyed. Notably, a harsh β-elimination can affect N-linked glycans.
Galactosyltransferase labeling is a powerful approach for product characterization and for identification of glycosylation sites and O-GlcNAc-modified proteins. Proteins labeled with [3H]Gal should first be separated from the majority of unincorporated label using size-exclusion chromatography (Fig. 5A). A negative control reaction should be used to identify the retention time of [3H]UDP-Gal. Fractions with signal higher than that of the blank should be combined and the protein precipitated (TCA, acetone, methanol). In the example shown, better resolution from the unincorporated label could be achieved by increasing the length of the column. Proteins are subsequently separated by SDS-PAGE and the [3H]Gal is detected by autoradiography. Ovalbumin is a useful control, as it both is labeled by galactosyltransferase and carries one glycan that is sensitive to PNGase F. Labeled proteins can be further assessed using a range of approaches such as product analysis (Basic Protocol 6), protein purification, and manual Edman degradation.
![Details are in the caption following the image Galactosyltransferase labeling. (A) Ovalbumin (pink squares) or a blank (black circles) were labeled with Gal-T and [<sup>3</sup>H]UDP-Gal. Reactions were desalted using a G50 column. Fractions were counted and those with significant signal above the blank were combined and acetone precipitated. (B and C) Ovalbumin (51,722 dpm) and HeLa cell lysates (18,295 dpm) labeled with Gal-T were separated by SDS-PAGE and stained with Coomassie blue (C: total protein). The resulting gel was dried and exposed to autoradiography film (1 week) with the aid of an intensifying screen (B).](https://static.yanyin.tech/literature_test/cpz1129-fig-0005-m.jpg)
O-GlcNAc-modified proteins can be enriched using antibody or lectin affinity chromatography (Basic Protocol 4; Lee et al., 2016). It should be noted that as these experiments are performed under native conditions, unmodified proteins that interact with O-GlcNAcylated proteins will also be enriched. Capturing O-GlcNAcylated proteins from nuclear and cytosolic lysates reduces the possibility of enriching proteins modified by N-linked glycans. When combining this approach with the Promega TNT system, fractions eluting from affinity columns should be subsequently separated by SDS-PAGE and detected by autoradiography to confirm that the radioactive signal arises from a protein with the appropriate molecular weight.
Basic Protocol 6 details conditions for detecting O-GlcNAc in 96-well plates that should be readily adapted for immunofluorescence. In Figure 6A, OGT wild-type and OGT null cells have been stained with CTD110.6. As expected, lower raw fluorescence signals are observed for O-GlcNAc in OGT null cells. As OGT null cells grow more slowly that OGT wild-type cells, the cell number is also reduced (Fig. 6A, middle panel; Kazemi et al., 2010). To generate the final data (Fig. 6A, lower panel), raw CTD110.6 signals were corrected for total cell number using the nuclear stain. In Figure 6B, U2OS cells treated with or without Thiamet-G have been stained using CTD110.6 resulting in a stronger signal. Similar to immunoblotting, incubation of the primary antibody with free GlcNAc provides an excellent specificity control. In Figure 6, incubation with free GlcNAc significantly reduces the signal. To generate final data, the raw fluorescence signal for CTD110.6 +100 mM GlcNAc is subtracted from the raw fluorescence signal for CTD110.6. Finally, as in Figure 6A, the specific CTD110.6 signal is corrected for cell number or total protein using a nuclear stain. Alternatively, the CTD110.6 signal could be normalized to total protein using a stain for a housekeeping protein such as actin.
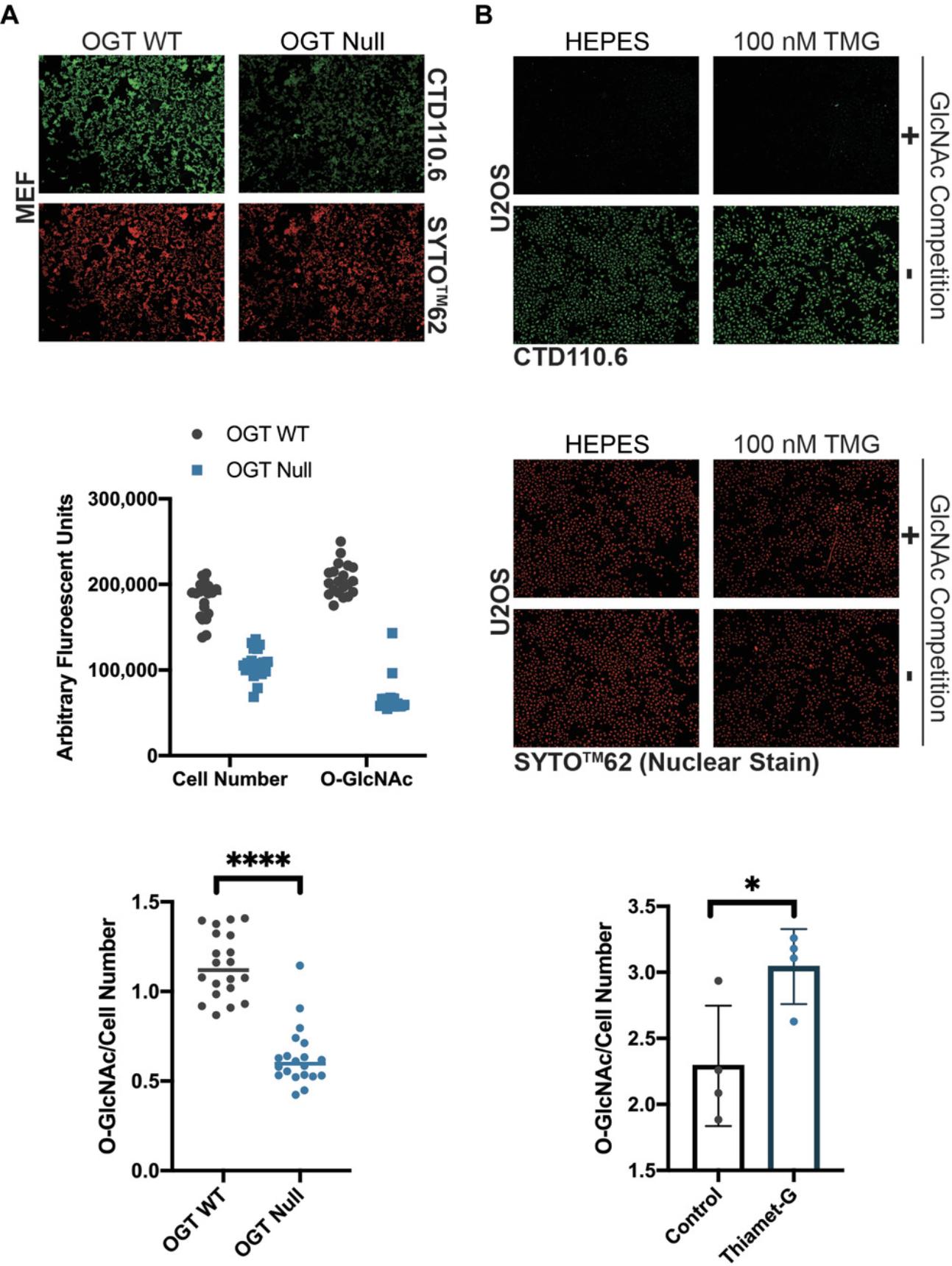
Figure 7 presents the raw data, an example calculation, and processed data for both OGT and OGA assays (Fig. 7A-D). To calculate OGT activity:
1.Triplicates are used to address variability and are averaged. 2.To assess autoglycosylation, a “no-peptide" control is used. OGT activity (dpm) = (signal from CK2 peptide) − (signal from no-peptide control). 3.To calculate DPM of GlcNAc added per minute per mg:
- Based on the specific activity of the [3H]UDP-GlcNAc, dpm values are converted to femtomoles of GlcNAc.
![Details are in the caption following the image Representative data from OGT and OGA assays. (A) OGT activity in 10 μg of desalted lysate from OGT wild-type (WT) and OGT null cells was assayed in triplicate in the presence and absence of the CK2 substrate peptide. The incorporation of [<sup>3</sup>H]GlcNAc was assessed using liquid scintillation counting (in dpm). Activity was subsequently calculated using 10 μg of protein, an assay length of 60 min, and 7.54 fmol of GlcNAc per 1000 dpm. (B) OGA activity in 10 μg of desalted lysate from OGT wild-type (WT) and OGT null cells was assayed in triplicate using the pseudosubstrates 4MU-β-GlcNAc and 4MU-β-GalNAc. One replicate was stopped using glycine at time = 0 (stopped). 4MU released was assessed in a fluorescence plate reader and quantified using a 4MU standard curve. Activity was subsequently calculated using 10 μg of protein, an assay length of 60 min, and 159,222 luminons per nanomole (experimentally derived standard curve). (C) Plotted OGT activity assays. (D) Plotted OGA activity assays. (E) Lysates from OGT WT and OGT null cells (10 μg) were separated by SDS-PAGE and electroblotted to nitrocellulose. The following were detected using a GE Healthcare AI600 RGB: Total Protein (Sypro™ Ruby Protein Blot stain), O-GlcNAc (CTD110.6), OGT (Sigma, DM17), and OGA (345; Butkinaree et al., 2008). Error bars represent one standard deviation. The p-values are the result of a Student's t-test (two-tailed). *p < 0.05, **p < 0.01.](https://static.yanyin.tech/literature_test/cpz1129-fig-0007-m.jpg)
Notably, a low signal-to-noise ratio suggests that the OGT assay buffer has expired or that the assay length or protein amount should be increased. Initial experiments should assess linear range through alteration of the amount of protein present in the assay. In this example, and as we expect, OGT activity is depressed in the OGT null cells.
With regard to OGA assays:
- 1.The reaction stopped at T = 0 min is used to assess the spontaneous breakdown of 4MU-GlcNAc/GalNAc and is background-subtracted from the average of the duplicates.
- 2.4MU-GalNAc is used to assess activity from lysosomal hexosaminidases. As such, to assess OGA activity, the averaged 4MU-GalNAc signal is background-subtracted from the 4MU-GlcNAc signal.
- 3.Finally, activity is expressed in nanomoles/minute/milligram by dividing the luminons by the assay length, adjusting for protein (here, multiplying by 100 because 10 μg of lysate was used), and then converted to nanomoles using an experimentally derived standard curve.
In this example, and as we expected, OGA activity was depressed in the OGT null cells. The example highlights importance of using the 4MU-GalNAc pseudo-substrate. First, readers will note that deletion of OGT that affects OGA abundance only significantly alters total luminons in the 4MU-GlcNAc samples. Secondly, readers will note that in spite of the GalNAc included in the assay, there is a significant difference between the 4MU-GalNAc replicates and the T = 0 sample. As for OGT assays, readers should ensure that they are within the linear range.
Time Considerations
Detection of O-GlcNAc proteins using antibodies (see Basic Protocol 2) and lectins (see Basic Protocol 3) will take ∼2-3 days after the extraction of the proteins from cells. Samples and controls must be treated with PNGase F and/or hexosaminidase (1 hr to overnight), before SDS-PAGE and blotting. In either case, overnight incubation at 4°C provides the best signal-to-noise ratio.
WGA affinity chromatography of low-copy-number proteins will take 2-3 days. The Promega TNT protein expression and WGA affinity chromatography can be completed in 1 day; subsequent analysis of the product by SDS-PAGE will take 1-2 days depending on the label used and the amount of label incorporated into proteins eluting from the WGA-agarose.
Autogalactosylation of the galactosyltransferase and subsequent analysis of the activity will take 1-2 days. As the enzyme is stable for 6-12 months at −20°C, autogalactosylation does not need to be repeated for each analysis. Galactosyltransferase labeling of the proteins can take several hours to overnight, though optimization of the conditions may take a few days. The subsequent analysis, as well as desalting (dependent on the technique used), PNGase F digestion (1 hr to overnight), precipitation of protein (3 hr to overnight), SDS-PAGE, and autoradiography (1-10 days), can take up to 2 weeks. The length of time allotted for product analysis is dependent on the methods chosen, but this will almost certainly require 7-10 days. Further analysis, including digestion of labeled proteins and subsequent purification of peptides, will take at least 3 days.
Similar to indirect immunofluorescence methods, the O-GlcNAc in-cell western blot using antibodies will take 2-3 days to complete following user-defined experiments. The O-GlcNAc primary antibodies require overnight incubation for efficient binding, but secondary antibody staining and analysis can be performed in a day.
OGT assays (Basic Protocol 7) are somewhat complicated and typically take 2 days to complete. Notably, after the OGT assay is quenched, it can be stored at −20°C for several weeks before desalting. O-GlcNAcase assays are straightforward and typically take 1 day.
Acknowledgments
Original research in the authors’ laboratories was supported by the U.S. National Institutes of Health (K12HL141952, U01 CA230978, and RO1HL139640 to N.E.Z.). G.W.H. receives a percentage of royalties received by the university on sales of the CTD 110.6 antibody. The terms of these arrangements are in accordance with the conflict of interest policy at The Johns Hopkins University.
Author Contributions
Kamau Fahie : Conceptualization, formal analysis, investigation, methodology, supervision, validation, writing-review and editing; Bhargavi Narayanan : Conceptualization, formal analysis, investigation, methodology, writing-review and editing; Fiddia Zahra : Formal analysis, methodology, writing-review and editing; Russell Reeves : Formal analysis, methodology, writing-review and editing; Gerald W. Hart : Funding acquisition, writing-original draft; Natasha E. Zachara : Conceptualization, funding acquisition, methodology, project administration, supervision, writing-original draft, writing-review and editing.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing not applicable—no new data generated.
Literature Cited
- Amano, J., & Kobata, A. (1989). Quantitative conversion of mucin-type sugar chains to radioactive oligosaccharides. Methods in Enzymology , 179, 261–270. doi: 10.1016/0076-6879(89)79127-8.
- Banerjee, S., Robbins, P. W., & Samuelson, J. (2009). Molecular characterization of nucleocytosolic O-GlcNAc transferases of Giardia lamblia and Cryptosporidium parvum. Glycobiology , 19, 331–336. doi: 10.1093/glycob/cwn107.
- Bonifacino, J. S., Dell'Angelica, E. C., & Springer, T. A. (2001). Immunoprecipitation. Current Protocols in Protein Science , 48, 10.16.1–10.16.6. doi: 10.1002/0471142727.mb1016s48.
- Boysen, R. I., & Hearn, M. T. (2001). HPLC of peptides and proteins. Current Protocols in Protein Science , 23, 8.7.1–8.7.40. doi: 10.1002/0471140864.ps0807s23.
- Brew, K., Vanaman, T. C., & Hill, R. L. (1968). The role of α-lactalbumin and the A protein in lactose synthetase: A unique mechanism for the control of a biological reaction. Proceedings of the National Academy of Sciences of the United States of America , 59, 491–497. doi: 10.1073/pnas.59.2.491.
- Brister, M. A., Pandey, A. K., Bielska, A. A., & Zondlo, N. J. (2014). OGlcNAcylation and phosphorylation have opposing structural effects in tau: Phosphothreonine induces particular conformational order. Journal of the American Chemical Society , 136, 3803–3816. doi: 10.1021/ja407156m.
- Butkinaree, C., Cheung, W. D., Park, S., Park, K., Barber, M., & Hart, G. W. (2008). Characterization of β-N -acetylglucosaminidase cleavage by caspase-3 during apoptosis. The Journal of Biological Chemistry , 283, 23557–23566. doi: 10.1074/jbc.M804116200.
- Caldwell, S. A., Jackson, S. R., Shahriari, K. S., Lynch, T. P., Sethi, G., Walker, S., … Reginato, M. J. (2010). Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene , 29, 2831–2842. doi: 10.1038/onc.2010.41.
- Cameron, A., Giacomozzi, B., Joyce, J., Gray, A., Graham, D., Ousson, S., … Hering, H. (2013). Generation and characterization of a rabbit monoclonal antibody site-specific for tau O-GlcNAcylated at serine 400. FEBS Letters , 587, 3722–3728. doi: 10.1016/j.febslet.2013.09.042.
- Chalkley, R. J., Thalhammer, A., Schoepfer, R., & Burlingame, A. L. (2009). Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proceedings of the National Academy of Sciences , 106, 8894–8899. doi: 10.1073/pnas.0900288106.
- Chou, T. Y., Hart, G. W., & Dang, C. V. (1995). c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. The Journal of Biological Chemistry , 270, 18961–18965. doi: 10.1074/jbc.270.32.18961.
- Comer, F. I., & Hart, G. W. (2001). Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry , 40, 7845–7852. doi: 10.1021/bi0027480.
- Comer, F. I., Vosseller, K., Wells, L., Accavitti, M. A., & Hart, G. W. (2001). Characterization of a mouse monoclonal antibody specific for O-linked N -acetylglucosamine. Analytical Biochemistry , 293, 169–177. doi: 10.1006/abio.2001.5132.
- Dennis, R. J., Taylor, E. J., Macauley, M. S., Stubbs, K. A., Turkenburg, J. P., Hart, S. J., … Davies, G. J. (2006). Structure and mechanism of a bacterial β-glucosaminidase having O-GlcNAcase activity. Nature Structural & Molecular Biology, 13, 365–371.
- Dong, D. L., & Hart, G. W. (1994). Purification and characterization of an O-GlcNAc selective N -acetyl-β-D-glucosaminidase from rat spleen cytosol. The Journal of Biological Chemistry , 269, 19321–19330. doi: 10.1016/S0021-9258(17)32170-1.
- Duk, M., Ugorski, M., & Lisowska, E. (1997). β-Elimination of O-glycans from glycoproteins transferred to immobilon P membranes: Method and some applications. Analytical Biochemistry , 253, 98–102. doi: 10.1006/abio.1997.9994.
- Elbaum, M. B., & Zondlo, N. J. (2014). OGlcNAcylation and phosphorylation have similar structural effects in α-helices: Post-translational modifications as inducible start and stop signals in α-helices, with greater structural effects on threonine modification. Biochemistry , 53, 2242–2260. doi: 10.1021/bi500117c.
- Elling, L., Zervosen, A., Gallego, R. G., Nieder, V., Malissard, M., Berger, E. G., … Kamerling, J. P. (1999). UDP-N -acetyl-α-D-glucosamine as acceptor substrate of β-1,4-galactosyltransferase. Enzymatic synthesis of UDP-N -acetyllactosamine. Glycoconjugate Journal , 16, 327–336. doi: 10.1023/A:1007039825505.
- Freeze, H. H. (2001). Lectin affinity chromatography. Current Protocols in Protein Science , 9.1.1–9.1.9.
- Fukuda, M. (1989). Characterization of O-linked saccharides from cell surface glycoproteins. Methods in Enzymology , 179, 17–29. doi: 10.1016/0076-6879(89)79110-2.
- Gallagher, S. (2001). Immunoblot detection. Current Protocols in Protein Science , 10.10–10.10.12.
- Gao, Y., Parker, G. J., & Hart, G. W. (2000). Streptozotocin-induced beta-cell death is independent of its inhibition of O-GlcNAcase in pancreatic Min6 cells. Archives of Biochemistry and Biophysics , 383, 296–302. doi: 10.1006/abbi.2000.2094.
- Gao, Y., Wells, L., Comer, F. I., Parker, G. J., & Hart, G. W. (2001). Dynamic O-glycosylation of nuclear and cytosolic proteins: Cloning and characterization of a neutral, cytosolic β-N -acetylglucosaminidase from human brain. The Journal of Biological Chemistry , 276, 9838–9845. doi: 10.1074/jbc.M010420200.
- Gloster, T. M., Zandberg, W. F., Heinonen, J. E., Shen, D. L., Deng, L., & Vocadlo, D. J. (2011). Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nature Chemical Biology , 7, 174–181. doi: 10.1038/nchembio.520.
- Greis, K. D., & Hart, G. W. (1998). Analytical methods for the study of O-GlcNAc glycoproteins and glycopeptides. Methods in Molecular Biology (Clifton, N.J.) , 76, 19–33.
- Greis, K. D., Hayes, B. K., Comer, F. I., Kirk, M., Barnes, S., Lowary, T. L., & Hart, G. W. (1996). Selective detection and site-analysis of O-GlcNAc-modified glycopeptides by beta-elimination and tandem electrospray mass spectrometry. Analytical Biochemistry , 234, 38–49. doi: 10.1006/abio.1996.0047.
- Gross, B. J., Kraybill, B. C., & Walker, S. (2005). Discovery of O-GlcNAc transferase inhibitors. Journal of the American Chemical Society , 127, 14588–14589. doi: 10.1021/ja0555217.
- Halim, A., Larsen, I. S. B., Neubert, P., Joshi, H. J., Petersen, B. L., Vakhrushev, S. Y., … Clausen, H. (2015). Discovery of a nucleocytoplasmic O-mannose glycoproteome in yeast. Proceedings of the National Academy of Sciences , 112, 15648–15653.
- Haltiwanger, R. S. (1998). Modulation of O -linked N -acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O -GlcNAc-β-N -acetylglucosaminidase inhibitor O -(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N -phenylcarbamate. Journal of Biological Chemistry , 273, 3611–3617. doi: 10.1074/jbc.273.6.3611.
- Haltiwanger, R. S., Blomberg, M. A., & Hart, G. W. (1992). Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N -acetylglucosamine: Polypeptide β-N -acetylglucosaminyltransferase. The Journal of Biological Chemistry , 267, 9005–9013. doi: 10.1016/S0021-9258(19)50380-5.
- Haltiwanger, R. S., Holt, G. D., & Hart, G. W. (1990). Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N -acetylglucosamine: Peptide β-N-acetylglucosaminyltransferase. The Journal of Biological Chemistry , 265, 2563–2568. doi: 10.1016/S0021-9258(19)39838-2.
- Han, C., Gu, Y., Shan, H., Mi, W., Sun, J., Shi, M., … Yu, W. (2017). O-GlcNAcylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nature Communications , 8, 1491. doi: 10.1038/s41467-017-01654-6.
- Han, I., Oh, E. S., & Kudlow, J. E. (2000). Responsiveness of the state of O-linked N -acetylglucosamine modification of nuclear pore protein p62 to the extracellular glucose concentration. Biochemical Journal , 350, 109–114. doi: 10.1042/bj3500109.
- Hardy, M. R., & Townsend, R. R. (1994). High-pH anion-exchange chromatography of glycoprotein-derived carbohydrates. Methods in Enzymology , 230, 208–225. doi: 10.1016/0076-6879(94)30014-3.
- Hart, G. W., Haltiwanger, R. S., Holt, G. D., & Kelly, W. G. (1989). Glycosylation in the nucleus and cytoplasm. Annual Review of Biochemistry , 58, 841–874. doi: 10.1146/annurev.bi.58.070189.004205.
- Hart, G. W., Slawson, C., Ramirez-Correa, G., & Lagerlof, O. (2011). Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annual Review of Biochemistry , 80, 825–858. doi: 10.1146/annurev-biochem-060608-102511.
- Hayes, B. K., Greis, K. D., & Hart, G. W. (1995). Specific isolation of O-linked N -acetylglucosamine glycopeptides from complex mixtures. Analytical Biochemistry , 228, 115–122. doi: 10.1006/abio.1995.1322.
- Heart, E., Choi, W. S., & Sung, C. K. (2000). Glucosamine-induced insulin resistance in 3T3-L1 adipocytes. American Journal of Physiology. Endocrinology and Metabolism , 278, E103–E112. doi: 10.1152/ajpendo.2000.278.1.E103.
- Holt, G. D., & Hart, G. W. (1986). The subcellular distribution of terminal N -acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. The Journal of Biological Chemistry , 261, 8049–8057. doi: 10.1016/S0021-9258(19)57510-X.
- Holt, G. D., Snow, C. M., Senior, A., Haltiwanger, R. S., Gerace, L., & Hart, G. W. (1987). Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N -acetylglucosamine. The Journal of Cell Biology , 104, 1157–1164. doi: 10.1083/jcb.104.5.1157.
- Isono, T. (2011). O-GlcNAc-specific antibody CTD110.6 cross-reacts with N -GlcNAc2-modified proteins induced under glucose deprivation. PLoS One , 6, e18959. doi: 10.1371/journal.pone.0018959.
- Izumi, T., & Suzuki, K. (1983). Neutral β-N -acetylhexosaminidases of rat brain. Purification and enzymatic and immunological characterization. The Journal of Biological Chemistry , 258, 6991–6999. doi: 10.1016/S0021-9258(18)32323-8.
- Jiang, J., Lazarus, M. B., Pasquina, L., Sliz, P., & Walker, S. (2011). A neutral diphosphate mimic crosslinks the active site of human O-GlcNAc transferase. Nature Chemical Biology , 8, 72–77.
- Kamemura, K., Hayes, B. K., Comer, F. I., & Hart, G. W. (2002). Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: Alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. The Journal of Biological Chemistry , 277, 19229–19235. doi: 10.1074/jbc.M201729200.
- Kazemi, Z., Chang, H., Haserodt, S., McKen, C., & Zachara, N. E. (2010). O-linked-N -acetylglucosamine (O-GlcNAc) regulates stress-induced heat sock protein expression in a GSK-3-dependent manner. Journal of Biological Chemistry , 285, 39096–39107. doi: 10.1074/jbc.M110.131102.
- Khidekel, N., Ficarro, S. B., Clark, P. M., Bryan, M. C., Swaney, D. L., Rexach, J. E., … Hsieh-Wilson, L. C. (2007). Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nature Chemical Biology , 3, 339–348. doi: 10.1038/nchembio881.
- Kim, E. J. (2018). Chemical reporters and their bioorthogonal reactions for labeling protein O-GlcNAcylation. Molecules (Basel, Switzerland) , 23, 2411. doi: 10.3390/molecules23102411.
- Kim, E. J., Kang, D. O., Love, D. C., & Hanover, J. A. (2006). Enzymatic characterization of O-GlcNAcase isoforms using a fluorogenic GlcNAc substrate. Carbohydrate Research , 341, 971–982. doi: 10.1016/j.carres.2006.03.004.
- Kobata, A. (1994). Size fractionation of oligosaccharides. Methods in Enzymology , 230, 200–208. doi: 10.1016/0076-6879(94)30013-5.
- Kreppel, L. K., Blomberg, M. A., & Hart, G. W. (1997). Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. The Journal of Biological Chemistry , 272, 9308–9315. doi: 10.1074/jbc.272.14.9308.
- Kreppel, L. K., & Hart, G. W. (1999). Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. The Journal of Biological Chemistry , 274, 32015–32022. doi: 10.1074/jbc.274.45.32015.
- Lee, A., Miller, D., Henry, R., Paruchuri, V. D. P., O'Meally, R. N., Boronina, T., … Zachara, N. E. (2016). Combined antibody/lectin enrichment identifies extensive changes in the O-GlcNAc sub-proteome upon oxidative stress. Journal of Proteome Research , 15, 4318–4336. doi: 10.1021/acs.jproteome.6b00369.
- Levine, P. M., Galesic, A., Balana, A. T., Mahul-Mellier, A.-L., Navarro, M. X., De Leon, C. A., … Pratt, M. R. (2019). α-Synuclein O-GlcNAcylation alters aggregation and toxicity, revealing certain residues as potential inhibitors of Parkinson's disease. Proceedings of the National Academy of Sciences , 116, 1511–1519. doi: 10.1073/pnas.1808845116.
- Liu, T.-W., Zandberg, W. F., Gloster, T. M., Deng, L., Murray, K. D., Shan, X., & Vocadlo, D. J. (2018). Metabolic inhibitors of O-GlcNAc transferase that act in vivo implicate decreased O-GlcNAc levels in leptin-mediated nutrient sensing. Angewandte Chemie International Edition , 57, 7644–7648. doi: 10.1002/anie.201803254.
- Lovrien, R. E., & Matulis, D. (2001). Selective precipitation of proteins. Current Protocols in Protein Science , 4.5–4.5.36.
- Ma, Z., & Vosseller, K. (2014). Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. The Journal of Biological Chemistry , 289, 34457–34465. doi: 10.1074/jbc.R114.577718.
- Macauley, M. S., Whitworth, G. E., Debowski, A. W., Chin, D., & Vocadlo, D. J. (2005). O-GlcNAcase uses substrate-assisted catalysis: Kinetic analysis and development of highly selective mechanism-inspired inhibitors. The Journal of Biological Chemistry , 280, 25313–25322. doi: 10.1074/jbc.M413819200.
- Martin, S. E. S., Tan, Z.-W., Itkonen, H. M., Duveau, D. Y., Paulo, J. A., Janetzko, J., … Walker, S. (2018). Structure-based evolution of low nanomolar O-GlcNAc transferase inhibitors. Journal of the American Chemical Society , 140, 13542–13545. doi: 10.1021/jacs.8b07328.
- Matsuoka, Y., Matsuoka, Y., Shibata, S., Yasuhara, N., & Yoneda, Y. (2002). Identification of Ewing's sarcoma gene product as a glycoprotein using a monoclonal antibody that recognizes an immunodeterminant containing O-linked N -acetylglucosamine moiety. Hybridoma and Hybridomics , 21, 233–236. doi: 10.1089/153685902760213831.
- Medina, L., Grove, K., & Haltiwanger, R. S. (1998). SV40 large T antigen is modified with O-linked N -acetylglucosamine but not with other forms of glycosylation. Glycobiology , 8, 383–391. doi: 10.1093/glycob/8.4.383.
- Monsigny, M., Sene, C., Obrenovitch, A., Roche, A. C., Delmotte, F., & Boschetti, E. (1979). Properties of succinylated wheat-germ agglutinin. European Journal of Biochemistry , 98, 39–45. doi: 10.1111/j.1432-1033.1979.tb13157.x.
- Moore, D. D. (2001). Commonly used stock solutions and reagents. Current Protocols in Molecular Biology , 35, doi: 10.1002/0471142727.mba02s35.
- Muha, V., Williamson, R., Hills, R., McNeilly, A. D., McWilliams, T. G., Alonso, J., … van Aalten, D. M. F. (2019). Loss of CRMP2 O-GlcNAcylation leads to reduced novel object recognition performance in mice. Open Biology , 9, 190192. doi: 10.1098/rsob.190192.
- Nandi, A., Sprung, R., Barma, D. K., Zhao, Y., Kim, S. C., Falck, J. R., & Zhao, Y. (2006). Global identification of O-GlcNAc-modified proteins. Analytical Chemistry , 78, 452–458. doi: 10.1021/ac051207j.
- Ngoh, G. A., Facundo, H. T., Hamid, T., Dillmann, W., Zachara, N. E., & Jones, S. P. (2008). Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circulation Research , 104, 41–49. doi: 10.1161/CIRCRESAHA.108.189431.
- Ortiz-Meoz, R. F., Jiang, J., Lazarus, M. B., Orman, M., Janetzko, J., Fan, C., … Walker, S. (2015). A small molecule that inhibits OGT activity in cells. ACS Chemical Biology , 10, 1392–1397. doi: 10.1021/acschembio.5b00004.
- Park, S.-K., Zhou, X., Pendleton, K. E., Hunter, O. V., Kohler, J. J., O'Donnell, K. A., & Conrad, N. K. (2017). A conserved splicing silencer dynamically regulates O-GlcNAc transferase intron retention and O-GlcNAc homeostasis. Cell Reports , 20, 1088–1099. doi: 10.1016/j.celrep.2017.07.017.
- Powell, L. D. (2001). Inhibition of N-linked glycosylation. Current Protocols in Protein Science , 12.3–12.3.9.
- Rao, X., Duan, X., Mao, W., Li, X., Li, Z., Li, Q., … Yi, W. (2015). O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nature Communications , 6, 8468–8477. doi: 10.1038/ncomms9468.
- Reeves, R. A., Lee, A., Henry, R., & Zachara, N. E. (2014). Characterization of the specificity of O-GlcNAc reactive antibodies under conditions of starvation and stress. Analytical Biochemistry , 457, 8–18. doi: 10.1016/j.ab.2014.04.008.
- Roos, M. D., Xie, W., Su, K., Clark, J. A., Yang, X., Chin, E., … Kudlow, J. E. (1998). Streptozotocin, an analog of N -acetylglucosamine, blocks the removal of O-GlcNAc from intracellular proteins. Proceedings of the Association of American Physicians , 110, 422–432.
- Roquemore, E. P., Chou, T. Y., & Hart, G. W. (1994). Detection of O-linked N -acetylglucosamine (O-GlcNAc) on cytoplasmic and nuclear proteins. Methods in Enzymology , 230, 443–460. doi: 10.1016/0076-6879(94)30028-3.
- Schindler, M., Hogan, M., Miller, R., & DeGaetano, D. (1987). A nuclear specific glycoprotein representative of a unique pattern of glycosylation. The Journal of Biological Chemistry , 262, 1254–1260. doi: 10.1016/S0021-9258(19)75779-2.
- Schnedl, W. J., Ferber, S., Johnson, J. H., & Newgard, C. B. (1994). STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes , 43, 1326–1333. doi: 10.2337/diab.43.11.1326.
- Shan, H., Sun, J., Shi, M., Liu, X., Shi, Z., Yu, W., & Gu, Y. (2018). Generation and characterization of a site-specific antibody for SIRT1 O-GlcNAcylated at serine 549. Glycobiology , 28, 482–487. doi: 10.1093/glycob/cwy040.
- Shanmugasundaram, B., Debowski, A. W., Dennis, R. J., Davies, G. J., Vocadlo, D. J., & Vasella, A. (2006). Inhibition of O-GlcNAcase by a gluco-configured nagstatin and a PUGNAc-imidazole hybrid inhibitor. Chemical Communications (Cambridge, England) , 42, 4372–4374. doi: 10.1039/B612154C.
- Shikhman, A. R., Greenspan, N. S., & Cunningham, M. W. (1993). A subset of mouse monoclonal antibodies cross-reactive with cytoskeletal proteins and group A streptococcal M proteins recognizes N -acetyl-β-D-glucosamine. Journal of Immunology (Baltimore, Md.: 1950) , 151, 3902–3913.
- Shikhman, A. R., Greenspan, N. S., & Cunningham, M. W. (1994). Cytokeratin peptide SFGSGFGGGY mimics N -acetyl-β-D-glucosamine in reaction with antibodies and lectins, and induces in vivo anti-carbohydrate antibody response. Journal of immunology (Baltimore, Md.: 1950) , 153, 5593–5606.
- Slawson, C., Zachara, N. E., Vosseller, K., Cheung, W. D., Lane, M. D., & Hart, G. W. (2005). Perturbations in O-linked β-N -acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. The Journal of Biological Chemistry , 280, 32944–32956. doi: 10.1074/jbc.M503396200.
- Snow, C. M., Senior, A., & Gerace, L. (1987). Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. The Journal of Cell Biology , 104, 1143–1156. doi: 10.1083/jcb.104.5.1143.
- Tan, Z.-W., Fei, G., Paulo, J. A., Bellaousov, S., Martin, S. E. S., Duveau, D. Y., … Walker, S. (2020). O-GlcNAc regulates gene expression by controlling detained intron splicing. Nucleic Acids Research , 48, 5656–5669. doi: 10.1093/nar/gkaa263.
- Tashima, Y., & Stanley, P. (2014). Antibodies that detect O-linked β-D-N -acetylglucosamine on the extracellular domain of cell surface glycoproteins. Journal of Biological Chemistry , 289, 11132–11142. doi: 10.1074/jbc.M113.492512.
- Teo, C. F., Ingale, S., Wolfert, M. A., Elsayed, G. A., Nöt, L. G., Chatham, J. C., … Boons, G.-J. (2010). Glycopeptide-specific monoclonal antibodies suggest new roles for O-GlcNAc. Nature Methods , 6, 338–343.
- Toleman, C. A., Schumacher, M. A., Yu, S.-H., Zeng, W., Cox, N. J., Smith, T. J., … Boyce, M. (2018). Structural basis of O-GlcNAc recognition by mammalian 14-3-3 proteins. Proceedings of the National Academy of Sciences , 115, 5956–5961. doi: 10.1073/pnas.1722437115.
- Torres, C. R., & Hart, G. W. (1984). Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. The Journal of Biological Chemistry , 259, 3308–3317. doi: 10.1016/S0021-9258(17)43295-9.
- Townsend, R. R., Basa, L. J., & Spellman, M. W. (1996). Identification and characterization of glycopeptides in tryptic maps by high-pH anion-exchange chromatography. Methods in Enzymology , 271, 135–147. doi: 10.1016/S0076-6879(96)71008-X.
- Townsend, R. R., & Hardy, M. R. (1991). Analysis of glycoprotein oligosaccharides using high-pH anion exchange chromatography. Glycobiology , 1, 139–147. doi: 10.1093/glycob/1.2.139.
- Townsend, R. R., Hardy, M. R., Cumming, D. A., Carver, J. P., & Bendiak, B. (1989). Separation of branched sialylated oligosaccharides using high-pH anion-exchange chromatography with pulsed amperometric detection. Analytical Biochemistry , 182, 1–8. doi: 10.1016/0003-2697(89)90708-2.
- Trinidad, J. C., Barkan, D. T., Gulledge, B. F., Thalhammer, A., Sali, A., Schoepfer, R., & Burlingame, A. L. (2012). Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Molecular & Cellular Proteomics, 11, 215–229.
- Turner, J. R., Tartakoff, A. M., & Greenspan, N. S. (1990). Cytologic assessment of nuclear and cytoplasmic O-linked N -acetylglucosamine distribution by using anti-streptococcal monoclonal antibodies. Proceedings of the National Academy of Sciences of the United States of America , 87, 5608–5612. doi: 10.1073/pnas.87.15.5608.
- Unverzagt, C., Kunz, H., & Paulson, J. C. (1990). High-efficiency synthesis of sialyloligosaccharides and sialoglycopeptides. Journal of the American Chemical Society , 112(25), 9308–9309. https://doi.org/10.1021/ja00181a037.
- Vosseller, K., Trinidad, J. C., Chalkley, R. J., Specht, C. G., Thalhammer, A., Lynn, A. J., … Burlingame, A. L. (2006). O-linked N -acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Molecular & Cellular Proteomics: MCP, 5, 923–934.
- Wells, L., Kreppel, L. K., Comer, F. I., Wadzinski, B. E., & Hart, G. W. (2004). O-GlcNAc transferase is in a functional complex with protein phosphatase 1 catalytic subunits. The Journal of Biological Chemistry , 279, 38466–38470. doi: 10.1074/jbc.M406481200.
- Whelan, S. A., Lane, M. D., & Hart, G. W. (2008). Regulation of the O-linked β-N -acetylglucosamine transferase by insulin signaling. The Journal of Biological Chemistry , 283, 21411–21417. doi: 10.1074/jbc.M800677200.
- Xu, S.-L., Chalkley, R. J., Wang, Z.-Y., & Burlingame, A. L. (2012). Identification of O-linked β-D-N -acetylglucosamine-modified proteins from Arabidopsis. Methods in Molecular Biology (Clifton, N.J.) , 876, 33–45. doi: 10.1007/978-1-61779-809-2_3.
- Yi, W., Clark, P. M., Mason, D. E., Keenan, M. C., Hill, C., Goddard, W. A., … Hsieh-Wilson, L. C. (2012). Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science , 337, 975–980. doi: 10.1126/science.1222278.
- Yuzwa, S. A., Macauley, M. S., Heinonen, J. E., Shan, X., Dennis, R. J., He, Y., … Vocadlo, D. J. (2008). A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nature Chemical Biology , 4, 483–490. doi: 10.1038/nchembio.96.
- Yuzwa, S. A., Shan, X., Macauley, M. S., Clark, T., Skorobogatko, Y., Vosseller, K., & Vocadlo, D. J. (2012). Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nature Chemical Biology , 8, 393–399. doi: 10.1038/nchembio.797.
- Yuzwa, S. A., Yadav, A. K., Skorobogatko, Y., Clark, T., Vosseller, K., & Vocadlo, D. J. (2011). Mapping O-GlcNAc modification sites on tau and generation of a site-specific O-GlcNAc tau antibody. Amino Acids , 40, 857–868. doi: 10.1007/s00726-010-0705-1.
- Zentella, R., Sui, N., Barnhill, B., Hsieh, W.-P., Hu, J., Shabanowitz, J., … Sun, T.-P. (2017). The Arabidopsis O-fucosyltransferase SPINDLY activates nuclear growth repressor DELLA. Nature Chemical Biology , 13, 479–485. doi: 10.1038/nchembio.2320.
- Zhu, Y., Shan, X., Yuzwa, S. A., & Vocadlo, D. J. (2014). The emerging link between O-GlcNAc and Alzheimer disease. The Journal of Biological Chemistry , 289, 34472–34481. doi: 10.1074/jbc.R114.601351.
Citing Literature
Number of times cited according to CrossRef: 4
- Wenhua Hu, Guolin Zhang, Yu Zhou, Jun Xia, Peng Zhang, Wenjin Xiao, Man Xue, Zhaohui Lu, Shuang Yang, Recent development of analytical methods for disease-specific protein O -GlcNAcylation , RSC Advances, 10.1039/D2RA07184C, 13 , 1, (264-280), (2023).
- Hirohito Abo, Masahiko Kume, Federico Pecori, Taichi Miura, Naoki Matsumoto, Shoko Nishihara, Kazuo Yamamoto, Disaccharide-tag for highly sensitive identification of O-GlcNAc-modified proteins in mammalian cells, PLOS ONE, 10.1371/journal.pone.0267804, 17 , 5, (e0267804), (2022).
- Duc Tan Huynh, Michael Boyce, Chemical Biology Approaches to Understanding Neuronal O−GlcNAcylation, Israel Journal of Chemistry, 10.1002/ijch.202200071, 63 , 1-2, (2022).
- Victoria Palin, Matthew Russell, Robert Graham, John D. Aplin, Melissa Westwood, Altered protein O-GlcNAcylation in placentas from mothers with diabetes causes aberrant endocytosis in placental trophoblast cells, Scientific Reports, 10.1038/s41598-021-00045-8, 11 , 1, (2021).

