Detecting reactive oxygen species in free-living symbiotic dinoflagellates exposed to nanoparticles
Liza M M Roger, Nastassja Lewinski
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
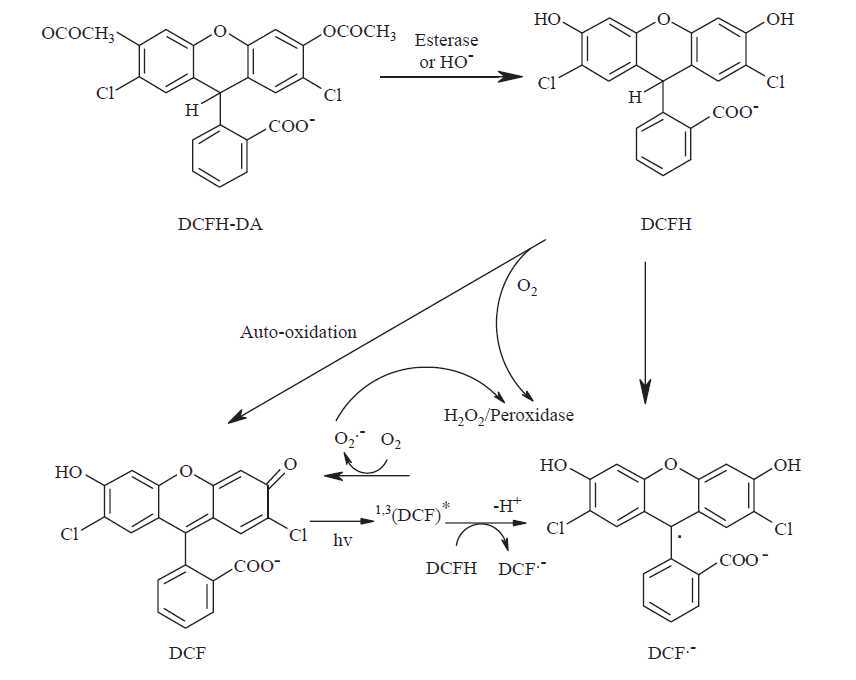
2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA) is a popular fluorescent probe for the detection of oxidative stress in cells. Since the probe can be prone to auto-oxidation, carboxy-2',7'-dichlorodihydrofluorescein-diacetate (carboxy-H2DCF-DA) which is more stable and easily penetrates cell membranes is often reported in the literature instead. Upon crossing the cell membrane, esterases hydrolyse DCFH-DA to DCFH, which remains trapped within cells. The oxidation of DCFH yields DCF, a fluorescent compound which can be measured using excitation/emission wavelengths of 485-495/520-530 nm.
Due to several cases of interference when used in cellular systems, DCFH-DA is a general marker of the cellular oxidative stress rather than a specific indicator H2O2 formation or other ROS. 2O2 formation or other ROS.
Steps
Chemicals
-
2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma-Aldrich, CAS Number 4091-99-0, MW 487.29, Product Number D6883)
-
Hydrogen peroxide (H2O2, 30%, Sigma-Aldrich, CAS Number 7722-84-1, MW 34.01, Product Number 216763)
-
BD DifcoTM Marine Broth 2216 (dehydrated culture media, Fisher Scientific, REF 279110)
-
Methanol (Fisher Chemical, A454-1, 0.2 micron filtered)
-
MilliQ water
-
Nanoparticle suspension
-
Cell suspension (here Breviolum minutum , wild type, marine dinoflagellate algae)
Equipment
-
15 mL conical tubes
-
50 mL conical tubes
-
1.5 mL or 2 mL Eppendorf tubes
-
Multichannel pipettor (at least 8 positions) with 300μL volume/pipette
-
1 mL pipettor and tips
-
20-200 μL pipettor and tips
-
10-100 μL pipettor and tips
-
0.5 to 2.5 μL pipettor and tips
-
96 well dosing plate, round bottom, sterile
-
96 well plate, black, sterile (note: clear 96-well plate can also be used but a black plate will prevent bleed-through between wells)
-
Troughs (reagent container)
-
Aluminium foil
-
Microplate reader
Free-living symbiotic dinoflagellate complete culture medium:
Dissolve 37.4 g of dehydrated BD DifcoTM Marine Broth 2216 in 1 L of MilliQ water.
Autoclave final solution.
Composition of BD DifcoTM Marine Broth 2216: https://www.fishersci.com/shop/products/bd-difco-dehydrated-culture-media-marine-broth-2216/DF0791174
H2O2 working solution:
Dilution 1: In a 2 mL Eppendorf tube, add 113 μL of stock solution of H2O2 and 886 μL sterile marine broth to achieve a final concentration of 1M H2O2.
Dilution 2: in a 2mL Eppendorf tube, add 30 μL of dilution 1 and 270 μL of sterile marine broth to achieve a final concentration 0.1M H2O2.
DCFH-DA solutions:
Stock solution: Dissolve 24.4 mg of DCFH-DA in 50mL methanol to make 1mM DCFH-DA concentrated stock solution.
This solution can be stored at -20°C for up to 4 months.
Working solution: in a 15 mL conical tube, add 100 μL of stock solution and 9.9 mL of sterile marine broth.
DCFH-DA loading and plating cells
In a 15 mL conical tube, add 6 mL of cell suspension (at 1x106 cells/mL) and 60 μL of DCFH-DA working solution. Wrap the tube in aluminium foil for 60 min dark incubation at room temperature. This is for half a 96-well plate at 150 μL per well (row B-G, columns 1-6)
After 60 min dark incubation, centrifuge the solution at 2000 rpm for 3 min at room temperature. Remove to supernatant and add 6 mL of fresh sterile marine broth. Plate cells in row B through G, columns 1 through 6 with 150 μL per well. Rows A and H (column 1 through 6) should be filled with 150 μL of sterile marine broth as blanks. Wrap plate in aluminium foil to prevent photoactivation or bleaching of DCFH, if you are not ready to dose the cells yet. Best practice would be to prepare the dosing plate during the DCFH-DA loading period so that you are ready to dose the cells as soon as the DCFH-DA loading is completed.
Nanoparticle working solution (CeO2)
The nanoparticles tested here are composed of CeO2 with a poly(acrylic acid) coating and DiI fluorescent labeling.
When synthesized, the colloid solution concentration was 1.3M.
To obtain the working solution:
-
dilution 1: in an Eppendorf tube, add 1 μL of colloid solution and 999 μL of marine broth;
-
dilution 2: in an Eppendorf tube, add 1 μL of dilution 1 and 999 μL of marine broth;
-
dilution 3: in an Eppendorf tube, add 10 μL of dilution 2 and 990 μL of marine broth (final concentration 3.6 μM)
Nanoparticle Dosing Plate prep
Prepare a nanoparticle working solution from your colloid solution (nanoparticles in suspension)to a final concentration of 1.3M. [Note: this protocol was developed for testing CeO2 nanoparticles and their potential for scavenging ROS in Breviolum minutum (symbiotic dinoflagellate often associated with Aiptasia anemones and Acropora corals)]
Preparation of the dosing plate:
-
in a round-bottom 96 well plate transfer 250 μL of complete sterile marine broth to column 1 to 6 in row B;
-
H2O2 replicates:
add 225 μL of sterile marine broth to columns 1 to 3 in row C;
add 225 μL of sterile marine broth to columns 1 to 3 in row D;
add 225 μL of sterile marine broth to columns 1 to 3 in row E;
add 225 μL of sterile marine broth to columns 1 to 3 in row F;
add 297 μL of sterile marine broth to columns 1 to 3 in row G;
add 3 μL of H2O2 working solution (dilution 2) to columns 1 to 3 in row G;
serial dilution: transfer 25 μL of wells G1-G3 to F1-F3 (using multichannel pipettor), repeat process from F1-F3 to E1-E3, from E1-E3 to D1-D3, from D1-D3 to C1-C3;
-
CeO2 replicates:
add 150 μL of sterile marine broth to columns 4 to 6 in row C;
add 125 μL of sterile marine broth to columns 4 to 6 in row D;
add 125 μL of sterile marine broth to columns 4 to 6 in row E;
add 125 μL of sterile marine broth to columns 4 to 6 in row F;
add 250 μL of CeO2 (3.6 μM in sterile marine broth) to columns 1 to 3 in row G:
serial dilution: transfer 125 μL of wells G4-G6 to F4-F6 (using multichannel pipettor), repeat process from F4-F6 to E4-E6, from E4-E6 to D4-D6, and 100 μL from D4-D6 to C4-C6;
Procedure
Centrifuge the 96-well plate with plated cells: 2000 rpm for 3 min at room temperature.
Remove supernatant (from columns 1 to 6, rows B to G) carefully using a fine tip plastic dropper pipette or standard pipettor;
[Be careful not to aspirate cells because the dinoflagellates do not attach]
Using a multichannel pipettor, transfer 150 μL from the dosing plate to the 96-well plate, well for well (from columns 1 to 6, rows B to G). Wrap the plate in aluminium foil until you analyze it in the plate reader. The analysis should be done directly after. The reaction starts as soon as you transfer the reagents from dosing plate to the plate with cells so the longer you wait the more of the reaction you miss.
Measure fluorescence of DCF using excitation/emission wavelengths of 485/530nm using a microplate reader at desired time points (every minute for up to 3H for kinetics or 1H for initial reaction);
Report peroxide production as the fluorescence intensity variation of treated cells relative to untreated cells;

