Detecting nitric oxide in free-living symbiotic dinoflagellates exposed to nanoparticles
Nastassja Lewinski, Liza M Roger
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
Diaminofuorescein-2 diacetate (DAF-2 DA) is a fluorescent indicator of nitric oxide (NO). The DAF reacts with the nitric anhydride (N2O3) which formed by oxidation of NO.
Upon crossing the cell membrane, esterases hydrolyse DAF-2 DA to DAF-2, which remains trapped within cells. The DAF-2 reacts to the oxidation of intracellular NO (or more accurately the N2O3) to produce the highly fluorescent triazolofluorescein (DAF-2T) by nitrosation and dehydration (Kojima et al., 1999).
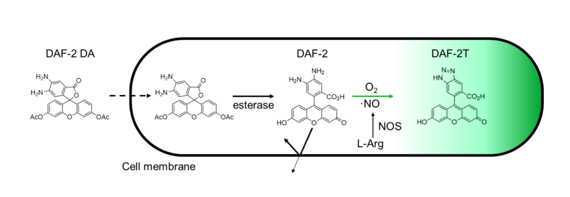
Protocol adapted from Bouchard & Yamasaki (2009) and Kojima et al. (1999)
Before start
DAF-2 DA information:
-
Prepare working solutions fresh if possible and protect from light;
-
Avoid repeated freeze-thaw cycles and wait until solution has reached room temperature before opening to avoid moisture from entering the vial;
-
Best practice would be to prepare and freeze aliquots when the chemical is first opened;
-
Addition of bovine serum albumin (BSA), phenol red, calcium ion and vitamins may affect the fluorescence.
Steps
Chemicals
-
Diaminofluorescein-2 diacetate (DAF-2 DA, C24H18N2O7, Sigma-Aldrich, CAS Number 205391-02-2, MW 446.4, SKU 251505-1MG-1)
-
Sodium nitroprusside dihydrate (SNP, Na2[Fe(CN)5NO] · 2H2O, Sigma-Aldrich, CAS Number 13755-38-9, MW 297.95, SKU 71778-25G)
-
Sterile Calcium-Magnesium free seawater (CMFSW)
-
MilliQ water
-
Nanoparticle suspension
-
Cell suspension (here Breviolum minutum , wild type, marine dinoflagellate algae)
Equipment
-
15 mL conical tubes
-
50 mL conical tubes
-
1.5 mL or 2 mL Eppendorf tubes
-
Multichannel pipettor (at least 8 positions) with 300μL volume/pipette
-
1 mL pipettor and tips
-
20-200 μL pipettor and tips
-
10-100 μL pipettor and tips
-
0.5 to 2.5 μL pipettor and tips
-
96 well dosing plate, round bottom, sterile
-
96 well plate, black, sterile (note: clear 96-well plate can also be used but a black plate will prevent bleed-through between wells)
-
Troughs (reagent container)
-
Aluminium foil
-
Microplate reader
Calcium-Magnesium free seawater (CMFSW) preparation:
Combine
-
23 g/L NaCl
-
0.763 g/L KCl
-
3 g/L NaSO4
-
0.25 g/L NaHCO3
with DI water and autoclave
Nanoparticle working solution:
The nanoparticles tested here are composed of CeO2 with a poly(acrylic acid) coating and DiI fluorescent labeling.
When synthesized, the colloid solution concentration was 1.3M.
To obtain the working solution:
-
dilution 1: in an Eppendorf tube, add 1 μL of colloid solution and 999 μL of CMFSW;
-
dilution 2: in an Eppendorf tube, add 1 μL of dilution 1 and 999 μL of CMFSW;
-
dilution 3: in an Eppendorf tube, add 10 μL of dilution 2 and 990 μL of CMFSW (final concentration 3.6 μM)
SNP working solution preparation:
Prepared a working solution of 3 mg/mL SNP in DI water (10 mM). Note: SNP is more soluble at >25°C.
Protect from light using aluminium foil
DAF-2 DA loading and plating cells
In a 15 mL conical tube, add 6 mL of cell suspension (at 1x106 cells/mL in CMFSW) and 9 μL of DAF-2 DA stock solution. Wrap the tube in aluminium foil for 60 min dark incubation at room temperature. This is for half a 96-well plate at 150 μL per well (row B-G, columns 1-6)
Note: it is important to use CMFSW because DAF- 2 DA reacts with calcium ions in typical seawater
After 60 min dark incubation, centrifuge the solution at 2000 rpm for 3 min at room temperature. Remove to supernatant and add 6 mL of fresh sterile CMFSW. Plate cells in row B through G, columns 1 through 6 with 150 μL per well. Rows A and H (column 1 through 6) should be filled with 150 μL of sterile CMFSW as blanks. Wrap plate in aluminium foil to prevent photoactivation or bleaching of DAF-2T, if you are not ready to dose the cells yet. Best practice would be to prepare the dosing plate during the DAF-2 DA loading period so that you are ready to dose the cells as soon as the DAF-2 DA loading is completed.
Nanoparticle dosing plate prep:
Prepare a nanoparticle working solution from your colloid solution (nanoparticles in suspension)to a final concentration of 1.3M. [Note: this protocol was developed for testing CeO2 nanoparticles and their potential for scavenging nitric oxide (NO) in Breviolum minutum (symbiotic dinoflagellate often associated with Aiptasia anemones and Acropora corals)]
Preparation of the dosing plate:
-
in a round-bottom 96 well plate transfer 250 μL of complete sterile marine broth to column 1 to 6 in row B;
-
SNP replicates:
add 225 μL of sterile CMFSW to columns 1 to 3 in row C;
add 225 μL of sterile CMFSW to columns 1 to 3 in row D;
add 225 μL of sterile CMFSW to columns 1 to 3 in row E;
add 225 μL of sterile CMFSW to columns 1 to 3 in row F;
add 297 μL of sterile CMFSW to columns 1 to 3 in row G;
add 3 μL of SNP working solution (dilution 2) to columns 1 to 3 in row G;
serial dilution: transfer 25 μL of wells G1-G3 to F1-F3 (using multichannel pipettor), repeat process from F1-F3 to E1-E3, from E1-E3 to D1-D3, from D1-D3 to C1-C3;
-
CeO2 replicates:
add 150 μL of sterile marine broth to columns 4 to 6 in row C;
add 125 μL of sterile marine broth to columns 4 to 6 in row D;
add 125 μL of sterile marine broth to columns 4 to 6 in row E;
add 125 μL of sterile marine broth to columns 4 to 6 in row F;
add 250 μL of CeO2 (3.6 μM in sterile marine broth) to columns 1 to 3 in row G:
serial dilution: transfer 125 μL of wells G4-G6 to F4-F6 (using multichannel pipettor), repeat process from F4-F6 to E4-E6, from E4-E6 to D4-D6, and 100 μL from D4-D6 to C4-C6;
Avoid direct exposure to light.
Procedure:
Centrifuge the 96-well plate with plated cells: 2000 rpm for 3 min at room temperature.
Remove supernatant (from columns 1 to 6, rows B to G) carefully using a fine tip plastic dropper pipette or standard pipettor;
[Be careful not to aspirate cells because the dinoflagellates do not attach]
Using a multichannel pipettor, transfer 150 μL from the dosing plate to the 96-well plate, well for well (from columns 1 to 6, rows B to G). Wrap the plate in aluminium foil until you analyze it in the plate reader. The analysis should be done directly after. The reaction starts as soon as you transfer the reagents from dosing plate to the plate with cells so the longer you wait the more of the reaction you miss.
Measure fluorescence of DAF-2T using excitation/emission wavelengths of 495/515nm using a microplate reader at desired time points (every minute for 1H);

