Detailed Western Blotting (Immunoblotting) Protocol
Rasheed Sule, Gabriela Rivera, Aldrin V Gomes
chemilumenescence
housekeeping proteins
ponceau S
stains free
Western bloting
Immunoblotting
gel electrophoresis
Abstract
Western Blotting, which is probably better referred to as immunoblotting, is one of the most commonly used biological methods worldwide. This technique is capable of detecting an individual protein from a complex mixture of proteins extracted from cells or tissues. The major steps in the Western Blotting workflow are 1) The separation of proteins based on size, 2) The transfer of separated protein to a suitable stable support, 3) Interaction between the target protein and appropriate primary antibodies. In many cases, a secondary antibody that interacts with the primary antibody is used, and 4) Visualization of the target protein using enhanced chemiluminescence (ECL), fluorescence, or colorimetric methods. A detailed protocol to help users get the most out of their Western Blots is presented.
Goal: To help Western Blotting users learn what prevents them from having perfect Western Blots
The most common result of your experiment:
A good Western blot.
Another possible result:
No signal detected or weak signal
Most likely reasons:
-
Antibody was not suitable (poor quality antibody).
-
Insufficent protein loaded on the gel for the amount of antibody used (or antibody too dluted).
-
Transfer efficiency from gel to membrane was poor.
-
ECL reagent was expired or contaminated.
-
Incorrect secondary antibody used.
-
Blocking agent concentration was too high.
For more reasons read [WESTERN BLOTTING TIPS AND TROUBLESHOOTING GUIDE](http://WESTERN BLOTTING TIPS AND TROUBLESHOOTING GUIDE)
Before start
Although the western blotting procedure is straightforward and well documented, problems can arise, leading to unexpected results.
A significant cause of problems is poor quality samples. For example, if samples were not stored properly before denaturation in sample buffer, many proteins may be proteolyzed and give artifactual results. Invest the time to ensure your samples are well prepared because, even if you do every other part of Western blotting perfectly, poor quality samples will result in inaccurate Western blots.
Steps
Sample preparation
Sample preparation can be simple or complex, depending on the source of the sample and the location of the target protein. It is recommended that you consult a dedicated article with procedures for optimal sample preparation.
This protocol assumes you have already prepared your sample and are ready to begin mixing with gel electrophoresis sample buffer.
Remove a small volume of sample to perform a protein quantification assay. Determine the protein concentration for each cell sample. Use a protein quantification appropriate method that is compatible with the reagents in your lysis/homogenization buffer.
Determine how much protein to load in each well and how many wells you will load. If you are going to load many wells on different gels over several weeks then prepare them at the same time. Once you know how much sample you need, dilute your sample appropriately or use it as is and add an equal volume of 2X Laemmli sample buffer to get the final concentration of your protein at a concentration you want. We recommend reducing and denaturing the samples unless the online antibody datasheet indicates that non-reducing and non-denaturing conditions should be used.
To reduce and denature your samples, heat each cell lysate in sample buffer at 75°C for 5 mins. Allow samples to cool and then use or aliquot and store at -20°C for future use (good for at least three months). For longer storage store at -80°C.
Loading and Running the Gel
Loading the sample is important as errors in loading will result in inaccurate results.
Prepare the electrophoresis tank by placing the gel inside the tank and fill with SDS-PAGE running buffer. Use the same pipette for loading all the wells and if the amount loaded should be the same then ensure that the level of the sample in each lane is the same by eye.
Load a molecular weight marker in appropriate wells of the SDS-PAGE gel, such as lane 1. Bio-Rad Precision Plus protein Standard Catalog #161-0374 (Bio-Rad Laboratories) works well.
We typically load 8-15 μg of total protein for cell lysates or tissue homogenates or 10–100 ng for purified protein. Larger amounts of protein can result in non-linearity when housekeeping proteins are used as normalization standards.
Run the gel for 1–2 hr at 120 V until the dye front reaches the agarose layer of the gel. Depending on the gel system you can run at higher voltages (consult your equipment manufacturer's guide).
When the dye front (caused by the bromophenol blue in the Laemmli buffer) reaches near the bottom or at the bottom of the gel, remove the gel from the glass or plastic plates. It is recommended that the wells be removed with a razor blade or similar object as this helps reduce your chance of having bubbles during transfer and makes handing of the gel easier.
Make a small cut at the top left side of the gel for orientation.
The gel percentage required is dependent on the size of your protein of interest:
| A | B |
|---|---|
| Protein size | Gel percentage |
| 4–40 kDa | 20% |
| 12–45 kDa | 15% |
| 10–70 kDa | 12.5% |
| 15–100 kDa | 10% |
| 25–100 kDa | 8% |
| 60-210 kDa | 5% |
Gel Percentage and Estimated Optimal Protein Separation Range
Gradient gels can also be used. .
| A | B |
|---|---|
| Protein size | Gel percentage |
| 5-200 kDa | 4-12 gradient |
| 4-200 kDa | 4-20 gradient |
| 3.5-110 kDa | 10-20 gradient |
Gel Percentage and Estimated Optimal Protein Separation Range
Transferring the protein from the gel to the membrane
The membrane can be either nitrocellulose or polyvinylidene fluoride (PVDF). There are a few differences between the two membranes:
| A | B |
|---|---|
| Nitrocellulose | PVDF |
| Less Expensive | More Expensive |
| Fast Protein Binding | Higher Sensitivity Protein Binding |
| Does Not Require Pre-Wetting | Requires Pre-Wetting with Methanol |
| Weaker Than PVDF | Stronger Than Nitrocellulose |
| Not Recommended for Stripping and Reprobing | Recommended for Stripping and Reprobing |
| Lower Protein Binding Capacity Than PVDF | Good for Hydrophobic Proteins |
| Requires Re-wetting with Methanol Throughout Use |
Advantages and disadvantages of the two most commonly used membranes for Western Blotting.
Activate PVDF with methanol for 1 min and rinse with transfer buffer before preparing the stack. Transfer of proteins to the membrane can be checked using Ponceau S staining before the blocking step.
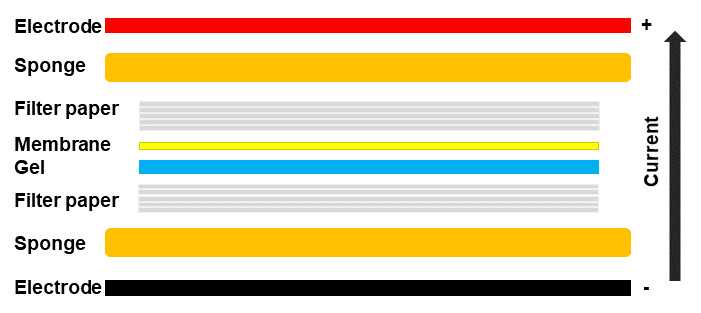
If using a wet or tank transfer, soak one stack of filter paper in transfer buffer and place it on the sponge. Wet the nitrocellulose membrane with transfer buffer and lay it on top of the filter paper stack. Allow at least 2 min for the filter paper and membrane to soak in the transfer buffer. Place the gel on top of the membrane, followed by another stack of filter paper soaked in transfer buffer.
Firmly roll out any air bubbles with a roller (such as a clean pencil). Use your fingers to apply mild pressure on the whole transfer stack while closing the cassette to ensure no additional air bubbles form. Once the stack has been completed, place the stack into the tank and fill the tank with transfer buffer. The tank should be run between 0.1 to 1 amps or 5 to 30 V for an hour or overnight, depending on the amp or voltage.
If using the Trans-blot Turbo (fast transfer), soak one stack of filter paper in transfer buffer and place it in the Trans-Blot Turbo Transfer System cassette. Wet the nitrocellulose membrane with transfer buffer and lay it on the filter paper stack. Allow at least 2 min for the filter paper and membrane to soak in the transfer buffer. Place the gel on the membrane, followed by another stack of filter paper soaked in transfer buffer. Firmly roll out the air bubbles with a roller. Use your fingers to apply mild pressure on the whole transfer stack while closing the cassette lid to ensure no additional air bubbles form. Wipe the outside of the cassette with tissue paper and place it inside the Trans-Blot Turbo Transfer System machine. Run with the appropriate setting. We use midi gels so our transfer uses the Turbo midi setting of 7 min to transfer the proteins from the gel to the membrane.
Take the membrane from the cassette and image it while still wet in the ChemiDoc MP Imaging System. If using Stain-Free gels, then use the Stain-Free membrane setting and optimized exposure time. There is no need to activate the membrane again. Adjust the exposure time as needed to avoid overexposed images. If necessary, the membrane can be cut into smaller sizes at this stage. If using normal gels use Ponceau S to stain the membranes.
Dry the membrane on top of a piece of tissue paper. Allowing the membrane to dry before proceeding to immunostaining has been suggested to help the proteins adhere better to the membrane.
Blocking
Blocking is an important step in the Western blotting process. Blocking prevents the non-specific binding of antibodies to the membrane.
Place the membrane in a blotting container and add enough blocking solution (3% Milk in TBST, or 5% BSA in TBST) to cover the entire membrane surface. Pour solutions into the corner of the blotting container and not directly onto the membrane so that the proteins on the membranes are not disturbed. Incubate the membrane on a shaker for 1 h. Pour out the blocking solution and rinse one time with TBST for 10-20 seconds to remove excess milk.
Primary Antibody Staining
The most critical part of any Western blot is the primary antibody.
Incubate the membrane with appropriate dilutions of primary antibody in blocking buffer (1% Milk in TBST, or 3 % BSA in TBST). We recommend overnight incubation at 4°C, or at least 4 hours incubation at room temperature.
Washing
Although it may seem like a trivial step, washing is important for improving the signal to noise (background) so that quantification is more accurate.
Wash the membrane in three washes of TBST, 3 min each.
Secondary Antibody
The choice of companies to purchase secondary antibodies is huge. Some companies have well established secondary antibodies that are respected and work well. We have used secondary antibodies from Cell Signaling, Bio-Rad, Sigma, and Abcam and they all worked well.
Incubate the membrane with the recommended dilution of conjugated secondary antibody in blocking buffer (1% Milk in TBST) at room temperature for 1 hr. For chemiluminescence, the secondary antibody is conjugated with horseradish peroxidase (HRP).
Washing Part II
Time to remove excess secondary antibodies.
Wash the membrane in three washes of TBST, 3 min each.
Detection
The choice of signal development reagents is expanding yearly. Most commercial preparation work well. Of 21 different commercial products we have tried, only one was disappointing.
For signal development, follow the kit manufacturer’s recommendations the first time you use their reagents.
Because of price and sensitivity, we utilize Clarity enhanced chemiluminescence (ECL) reagent. The amount of ECL reagent depends on the size of the blot. For a 3 cm x 4 cm membrane strip, we use 1 ml of ECL reagent. In a 1.5 mL centrifuge tube, mix equal parts of Clarity western peroxide agent (0.5 mL) and Clarity western luminol/enhancer reagent (0.5 mL). This creates the substrate for chemiluminescence. Pipette enough ECL substrate to evenly cover the surface of the membrane where the proteins are attached. Incubate the ECL covered membrane in a dark condition for 2 min before removing the excess ECL reagent.
Imaging
Pick up the membrane from one side with a pair of blunt forceps and drain the excess ECL reagent by gently touching the edge of the membrane to a delicate tissue paper. Arrange the membrane on the imaging surface, taking care not to create bubbles on the surface.
Image the blot on your machine of choice. We utilize the Bio-Rad ChemiDoc MP Imaging system with the Chemi Hi Sensitivity setting and 10 seconds of imaging time initially. If the image is saturated (red bands) then we use the Chemi Hi-Resolution setting with the optimized exposure time. If the initial 10 s image was not visible, we do a 60 s high sensitivity image.
The exposure time can be adjusted as needed to obtain the best image that contains no overexposed (saturated) bands.
One of the advantages of the ChemiDoc MP is that we are able to take a multichannel image with the stain-free and either the Chemi Hi Sensitivity or Hi-Resolution setting to get an image of the chemiluminescent protein bands of interest overlaid with the stain-free prestained protein markers.
Although the other proteins on the membrane will also show up with the stain-free image, the chemiluminescent signal shows up on a different channel. Verify the molecular weight of the band of interest based on the multichannel image.
After you get your great looking blots you may want to quantify your results.
A major concern of western blot quantification is the difficulty associated with determining saturated signals. This is especially problematic when using film. It is recommended that a digital imager be used (many universities now have digital imagers so try to find a lab that has one). Please read the manufacturer’s recommendation on how to use the specific imager you are using as well as do a google search for problems with quantification using the software that you will use to quantify. Unfortunately, after getting great blots some researchers do inaccurate quantification of their results. Invest your time to learn how to quantify the signals accurately.
Relax
Sit back and enjoy your Western blotting results.