Creating pooled CRISPR-Cas9 knock-outs in NIH-3T3 cells
Suzanne R Pfeffer, Herschel Dhekne, Ebsy Jaimon
Abstract
To validate a genome wide CRISPR screen, we select the top hits and create lentiviruses to validate the hits. Rather than screening each virus from single cell clones, we analyze the infected cells as pools according to the following protocol. This method involves three major steps:
- Create Cas9-expressing 3T3 cells (1-3 months)
- Make lentiviruses that carry sgRNA (2 weeks)
- Infect 3T3-Cas9 cells and assess gRNA efficiency (1 week)
Steps
Create 3T3-Cas9 cells and Clone the CRISPR guides
Generate 3T3-Cas9 cells
Infect NIH3T3-flpin cells with Cas9-containing lentivirus to generate cells constitutively expressing Cas9 as follows:
- Plate NIH-3T3 cells into a 6 well cell dish at 1x105 cells / well
- After cells attach, add lentivirus made from Lenti-Cas9 vector onto the cells (dx.doi.org/10.17504/protocols.io.bp2l61z2zvqe/v1)
- After
48h 0m 0s, trypsinize cells and plate into 3 wells of a 6 well plate in media containing 10µg/ml Blasticidin - Cells infected with Lenti-Cas9 will survive Blasticidin and
72h 0m 0safter treatment, selection is stopped and Blasticidin-resistant survivors are expanded in normal medium
- Cas9 is toxic to these cells and they grow slower than the parental cells; These cells are more sensitive to cell death than usual and care must be taken to passage them regularly at 80% confluency. Change medium every 48 hours and avoid high passage numbers
- At this point, test cells for Cas9 expression by western blot using mouse anti-Cas9 antibody (Biolegend 844302)
- Sort 3T3-Cas9 cells to obtain single cells in a 96 well plate using a cell sorter; Allow cells to grow for 3 weeks with occasional medium exchange
- Cells that are grown in 25% conditioned medium (previous 3-day old 3T3 medium passed through a 0.2µm filter) grow faster
- Glutamax instead of Glutamine also helps cells grow faster
- Transfer clones that grow into 24 well plates and a few days later, into 6 well plates
- Expand each clone and freeze cells in liquid nitrogen
Identify the best 3T3 Cas9 clones
Healthy clones appear fibroblast-like, without vacuoles, and grow at a rate such that they need a 1:5 split every 3 days. Expand such clones and test for Cas9 expression by lysing in RIPA buffer and performing a western blot using anti-Cas9 antibody
Retain cells expressing the highest Cas9 and marker of choice (in our case, phospho-Rab10); of those, select clones that have the best growth and morphology
Test the top two clones for Cas9 activity by introducing a validated sgRNA known to produce knock-outs efficiently as follows:
- Plate each 3T3 cell clone in wells of a 6 well plate (as above)
- Infect cells 8 h after plating with a lentivirus carrying sgRNA targeting mouse GRK2 (Pusapati et al. 2018) that is known to knock-out GRK2 efficiently; perform an additional mock infection as a control
- After , remove the virus supernatant, trypsinize cells and split cells into 2 wells of a 6 well plate and culture with 1µg/ml Puromycin. Cells not infected with the gRNA lentivirus will die within
72h 0m 0swhile resistant survivors grow - Exchange the growth medium with puromycin-free medium to allow cell expansion; depending on the virus titre, allow 3 days for cells to grow
- At confluency, lyse cells and test the knock-out efficiency by western blot using a mouse anti-target antibody, in this case, anti-GRK2 antibody
#尊敬的用户,由于网络监管政策的限制,部分内容暂时无法在本网站直接浏览。我们已经为您准备了相关原始数据和链接,感谢您的理解与支持。
https://lh4.googleusercontent.com/53Ob0YXc1DOOyI71XwhPxCZFlMdTUiV60H_eW9dIhR20rirsHO_nQgyyvVp8G8G1qOykdCBY3Mgq1IAathMalsekK830ct026xz33ta9-FRBcXKYVBZMt6h5CJD3XxG5-SzINoz8e3v_GvQmyw783GeIYLTnrY1lzztx5PydSllKvygpTLWZwWU5Pm8AMg
#尊敬的用户,由于网络监管政策的限制,部分内容暂时无法在本网站直接浏览。我们已经为您准备了相关原始数据和链接,感谢您的理解与支持。
https://lh6.googleusercontent.com/E3Jv4TfbnonCsSLWcyCYoo60EXyKU97fxe94CfOUcmZwdz1s9et2gJ46nIl4cQQq8FCImA6lqm4xf_Ka8NJx1DN5F9uvmCQWNC5fPidfiw6eaSF7tmeYcmSm2hvk5dHMwrONO3NgYMHtwnFcsHOTrHLUxDkYaVBj9uJkzJhAym0Ms9kbqTCNlacyp1u4Qg
Clone gRNA Vector
-
Digest Lenti-guide puro using Esp3I or BsmB1-V2 enzyme as follows: (Lenti-CRISPR-V2 can also be digested using the same enzyme)
| A | B |
|---|---|
| Reagent: | |
| Tango buffer 10X | 5µl |
| Lentiguide puro vector (500ng/µl) | 10µl |
| Esp3I or Bsmb1-V2 | 5µl |
| DTT 0.1M | 2µl |
| Water | Up to 50µl |
- Digest overnight at 37˚C
- A 2kb insert is generated by this cleavage; excise the upper band of the digested vector from the gel using a clean razor blade. Carry out standard DNA extraction from the agarose gel
- Dilute the cut vector to 20ng/µl and store at -20°C for further use
Prepare the Insert
- Order oligonucleotides to clone a guide RNA sequence using Addgene’s protocol on the lenti-guide puro webpage (https://media.addgene.org/data/plasmids/52/52963/52963-attachment_IPB7ZL_hJcbm.pdf). These oligos can alternatively be cloned into Lenti-CRISPR-v2
- Briefly, for each guide, two reverse complement oligos are ordered such that :
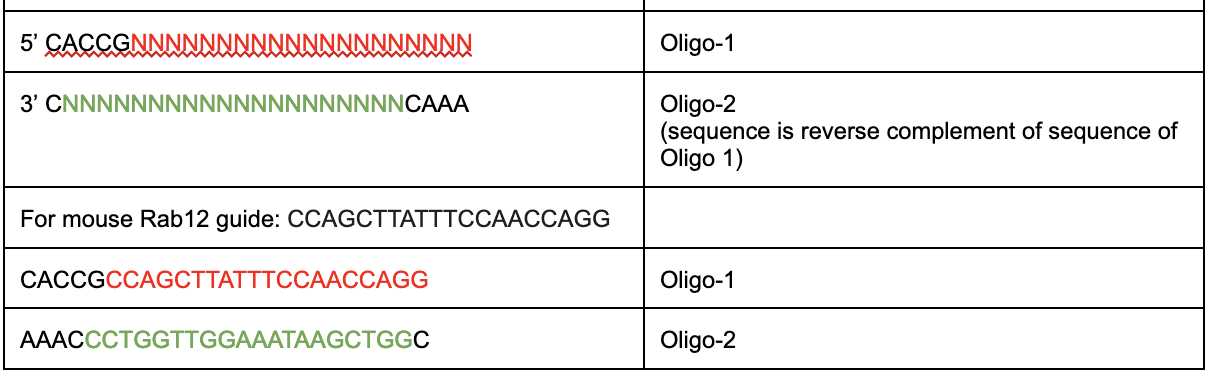
- Each oligo is diluted to 10µM and annealed and phosphorylated in a PCR tube as follows:
| A | B |
|---|---|
| Reagent | Volume |
| 10X T4 ligase buffer | 2µl |
| Oligo 1 (10µM) | 2µl |
| Oligo 2 (10µM) | 2µl |
| T4 PNK | 0.5µl |
| Water | 13.5µl |
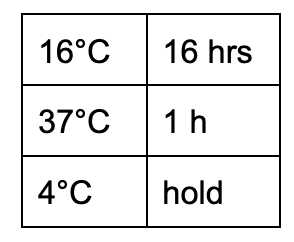
- Transfer to a PCR machine
| A | B |
|---|---|
| Reagent | Volume |
| Hold at 37°C | 60 min |
| Ramp up to 95°C (fast mode) | |
| 95°C | 3 min |
| Ramp down to 25°C | 0.2°C/sec |
| 25°C | 10 min |
| 4°C | hold |
- Incubate in a PCR machine as follows:
| A | B |
|---|---|
| Reagent | Volume |
| T4 ligase buffer 10x | 1µl |
| Vector (20ng) | 1µl |
| Insert (from previous step) | 1µl |
| T7 ligase | 1µl |
| 0.1M ATP | 0.5µl |
| 0.1M DTT | 0.5µl |
| Water | 5µl |
- Transform 5µl of the 10µl reaction into Dh5alpha competent cells
- Plate onto carbenicillin agar plates overnight
- To assess cloning efficiency, perform a colony PCR on the colonies 18 hr after transformation using the primers:
| A | B |
|---|---|
| Forward | 5’ ATCATATGCTTACCGTAACTTGAAAGTATTTCG |
| Reverse | 5’ GACTCGGTGCCACTTTTTCAAG |
- Pick a colony using a sterile tip and dilute into 10µl of Luria broth in a PCR tube
- Amplify a plasmid that has a guide cloned into the vector as a positive control
- Amplify a Bsmb1 digested plasmid as a negative control
- Amplify a colony from a control plate that didn’t receive an insert as a negative control
- Amplify the uncut plasmid as a negative control
These are the conditions for amplification of these plasmids:
| A | B |
|---|---|
| Green GoTaq master mix 2X | 12.5µl |
| Primer-Forward(10µM) | 1µl |
| Primer-Reverse(10µM) | 1µl |
| Bacterial suspension (diluted into 10µl) | 1µl |
| Water | 9.5µl |
| A | B | C |
|---|---|---|
| 95°C | 3 min | |
| 95°C | 30 sec | Cycle 35 times |
| 55°C | 30sec | |
| 72°C | 20 sec | |
| 72°C | 1 min |
- The green GoTaq PCR mix is ready to load onto a 2% agarose gel immediately after PCR
- The correct sized product is ~166bp
- Don’t forget to use a positive control (a cloned gRNA vector - 166bp band) and a negative control (uncut original lenti guide puro vector - 2kb band)
- Dilute the crude PCR product 1:10 and send for Sanger sequencing using the forward or reverse primer used for colony PCR
- Grow colonies that show the right sized band overnight at 37°C in 50µg/ml carbenicillin and perform a plasmid mini-prep
- Use the cloned plasmid for small scale lentivirus generation (dx.doi.org/10.17504/protocols.io.bp2l61z2zvqe/v1) if the Sanger sequencing shows the correct gRNA sequence has been cloned into the vector
Create pooled knock-out 3T3 cells
Plate 1x105 3T3-Cas9 cells in one well of a 6 well plate containing 2ml complete DMEM medium (10% fetal bovine serum, penn/strep and Glutamax)* After 6 hours, add 0.5ml gRNA lentivirus plus 4µg/ml polybrene
- After 48 h, trypsinize and split the cells into 2 wells of a 6 well plate with 1 µg/ml puromycin
- After an additional 48-72 h, exchange the medium with complete DMEM without puromycin and let the cells grow for 2 more days
- At 80% confluence, trypsinize and plate 5x104 cells on coverslips in each well of a 24 well plate
- At the same time, take 20µl of the trypsinized cells and mix in 20µl QuickExtract solution to extract genomic DNA for knock out genotyping
- Perform QuickExtract genomic extraction for parental wild type 3T3 and lentivirus-gRNA infected cells
- Extract DNA according to this modified manufacturer’s protocol | A | B | | --- | --- | | 65°C | 6 min | | 98°C | 10 min | | 4°C | hold |
(Some protocols suggest 65°C for 10 min and that also works)
- PCR amplify a region around the gRNA-Cas9 cut site using genotyping PCR primers designed such that ~300-500bp are covered around the guide target site
For the gRNA used in the above example targeting mouse Rab12:
| A | B |
|---|---|
| Forward | CCAAGGCCATGGTCATTCTTGATG |
| Reverse | TTACAACCCCAAACACTTGTTCCG |
| A | B |
|---|---|
| GoTax master mix 2X | 12.5µl |
| Primer-Forward (10µM) | 1µl |
| Primer-Reverse (10µM) | 1µl |
| Quick Extracted genomic DNA | 2µl |
| Water | 8.5µl |
| A | B | C |
|---|---|---|
| 95°C | 2 min | |
| 95°C | 30 sec | 35 cycles |
| 55°C | 30 sec | |
| 72°C | 45 sec | |
| 72°C | 1 min |
If the PCR band is of the correct size, dilute the sample 1:10 and Sanger sequence the crude PCR product using the reverse primer from the PCR
-
Perform TIDE analysis (www.tide.nki.nl) or Synthego ICE analysis using the raw chromatograms in the .abi file provided with the sequencing results
-
Percentage of cells with insertions or deletions (INDELs) is a proxy for knock-out efficiency
-
The higher the percentage, the better the knock-out efficiency
-
If needed, cells can be single cell sorted to create clonal knock-out lines for long term studies of the phenotype
-
For 3T3 cells, increased passage number can be detrimental to cell physiology
-
Best gRNAs with highest INDEL percentages are analyzed for the desired phenotype(s)
-
Using the above mentioned gRNA directed against mouse Rab12, within 5 days we observed 80% efficiency
-
Cells can be single cell sorted into 96 well plate using a Sony cell sorter at this point to create clonal cell lines with gene knock-outs

