Correlative Microscopy for Localization of Proteins In Situ: Pre-embedding Immuno-Electron Microscopy Using FluoroNanogold, Gold Enhancement, and Low- Temperature Resin
Daniela Boassa
Pre-embedding immuno-EM
FluoroNanogold
Gold enhancement
LR White
Normal rat kidney
Correlative microscopy
Fluorescence microscopy
Electron microscopy
UCSD
NCMIR
Abstract
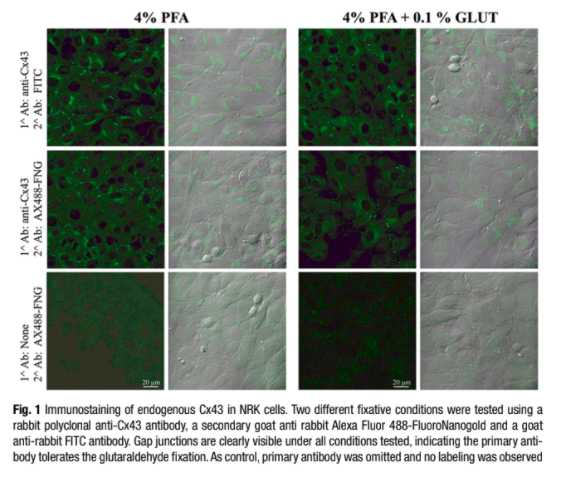
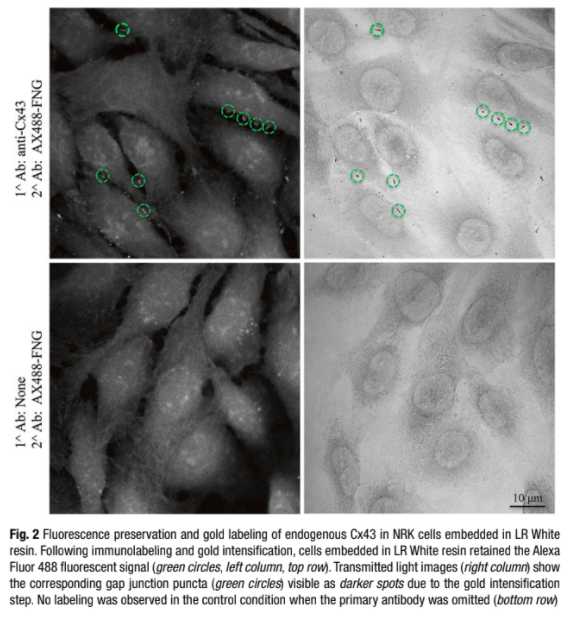
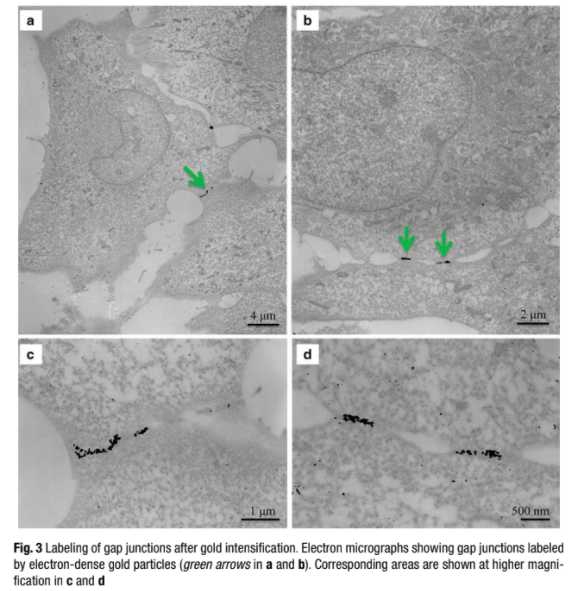
Immuno-electron microscopy (immuno-EM) is a technique that has been used widely to determine sub cellular localization of proteins. Different approaches are available for immuno-EM: pre-embedding method, post-embedding, and cryosectioning (Tokuyasu “style”). Here we describe a pre-embedding technique that allows the labeling of a target protein in situ , retention of fluorescence signal in plastic, and its localization at the EM level in a given cellular context. The procedure can be technically challenging and labor intensive: it requires optimization of fixation protocols to better preserve the cellular morphology and screening of compatible antibodies. Nevertheless, immuno-EM can be a powerful localization tool.
Before start
Perform a fixation series of paraformaldehyde and glutaraldehyde dilutions, and assess where the fluorescence signal is lost.
Steps
Remove the culture medium from the MatTek dishes contain ing the cells and wash three times with HBSS pre-warmed up at 37 °C. Fix cells with pre-warmed fixative at 37 °C (4 % PFA, 4 % PFA + 0.1 % glut, 2 % PFA, 2 % PFA + 0.1 % glut) for 5 min at room temperature followed by 30 min over ice. All solutions and steps from this point on are utilized and performed at 4 °C for the preservation of the ultrastructure (see Safety Warnings).
Remove fixative and wash cells three times for 2 min each in ice-cold 1× PBS. Note: during any washing step the cells should never become dry to avoid irreversible damage to morphology.
Incubate in blocking buffer for 1 h at 4 °C (see Guideline 1).
Incubate in primary antibody diluted in blocking buffer at 4 °C for 1 h on a rocking platform set for gentle agitation (see Guideline 2).
Rinse cells with Working Buffer, six times for 2 min each at 4 °C.
Incubate in secondary antibody diluted in blocking buffer at 4 °C for 1 h on a gentle rocker, protected from light. Remember: keep cells in the dark from now on, to preserve fluorescence.
Rinse cells with Working Buffer, six times for 3 min each at 4 °C.
Wash cells with ice-cold 1× PBS, three times for 3 min each at 4 °C.
Postfix with 2 % glutaraldehyde in PBS for 10 min at 4 °C.
Remove fi xative and rinse three times for 2 min each in ice-cold 1× PBS. Then wash cells for 5 min in quenching solution at 4 °C to remove aldehydes (see Guideline 3).
Rinse three times for 5 min each in ddH2O at 4 °C.
Gold enhancement : incubate cells in the Gold Enhancement kit for 1–5 min to intensify gold particles. The reaction is light insensitive so it can be carried out under normal room lighting.
The kit is composed of 4 components: the enhancer (A),the activator (B), the initiator (C), and the buffer (D).
Equilibrate the solutions at room temperature. Right before use, combine equal volumes of A and B first, wait 5 min then add equal amounts of C and D (see Guideline 4).
Thoroughly rinse three times for 5 min each in ddH2O at 4 °C.
Dehydration (ethanol series): dehydrate in increasing concentration of ethanol: 20, 50, 70, 90, and 2× 100 % ice-cold ethanol for 2 min each. Then rinse with 100 % ethanol at room temperature for 2 min.
Infiltration and embedding:
-
Remove 100 % ethanol and replace with a 50:50 mixture of low-temperature resin (LR White): ethanol 100 % for 20 min at room temperature.
-
Remove the mixture and replace with 100 % LR White for 1 h. MatTek dish can be stored overnight in unpolymerized resin at 4 °C.
-The following day allow three more changes in 100 % of LR White within an hour.
Cold-cure procedure: In a glass vial add 10 ml of LR White and one drop of accelerator. Mix well but avoid introducing any air to the solution and immediately add to cells. Cover with a piece of Aclar Embedding fi lm, avoiding any air bubbles. Place MatTek dish inside an aluminum dish with ethanol on ice to keep the temperature low. Keep it on ice until the resin polymerizes (30 min to an hour).
Store at 4 °C.