Construction of individuals ddRADseq libraries for macro-algae (Kelp) V.3
Stéphane Mauger, Komlan Avia
Abstract
This protocol describes a double digested restriction-site associated DNA (ddRADseq) procedure, that is a variation on the original RAD sequencing method (Davey & Blaxter 2011), which is used for de novo SNP discovery and genotyping.
This protocol differs from the original ddRADseq protocol (Peterson et al 2012), in which the samples are pooled just after the ligation to adaptors (i.e. before size selection and PCR). This protocol is an update of the protocol from Claire Daguin Thiebaut et al. (dx.doi.org/10.17504/protocols.io.bv4tn8wn) adapted for macro-algae.
The following protocol is intended for the construction of individual ddRADseq libraries from genomic DNA of various macro-algae samples (Kelp). In the present protocol, we added a genomic DNA purification step to eliminate the inhibitors of PCR and Ligation present in macro-algae (polysaccharides). Moreover, all samples are treated separately until final PCR amplification (Rad Taq enrichment step) performed before pooling.
Despite being slightly more costly and time-consuming in the lab, it allows for fine adjustement of each sample representation in the final library pool ensuring similar number of reads between samples. Finally, we have defined new P1 adapters (barcodes) with variable sequences and variable sizes (6bp to 13bp) to increase the efficiency of the Illumina sequencing.
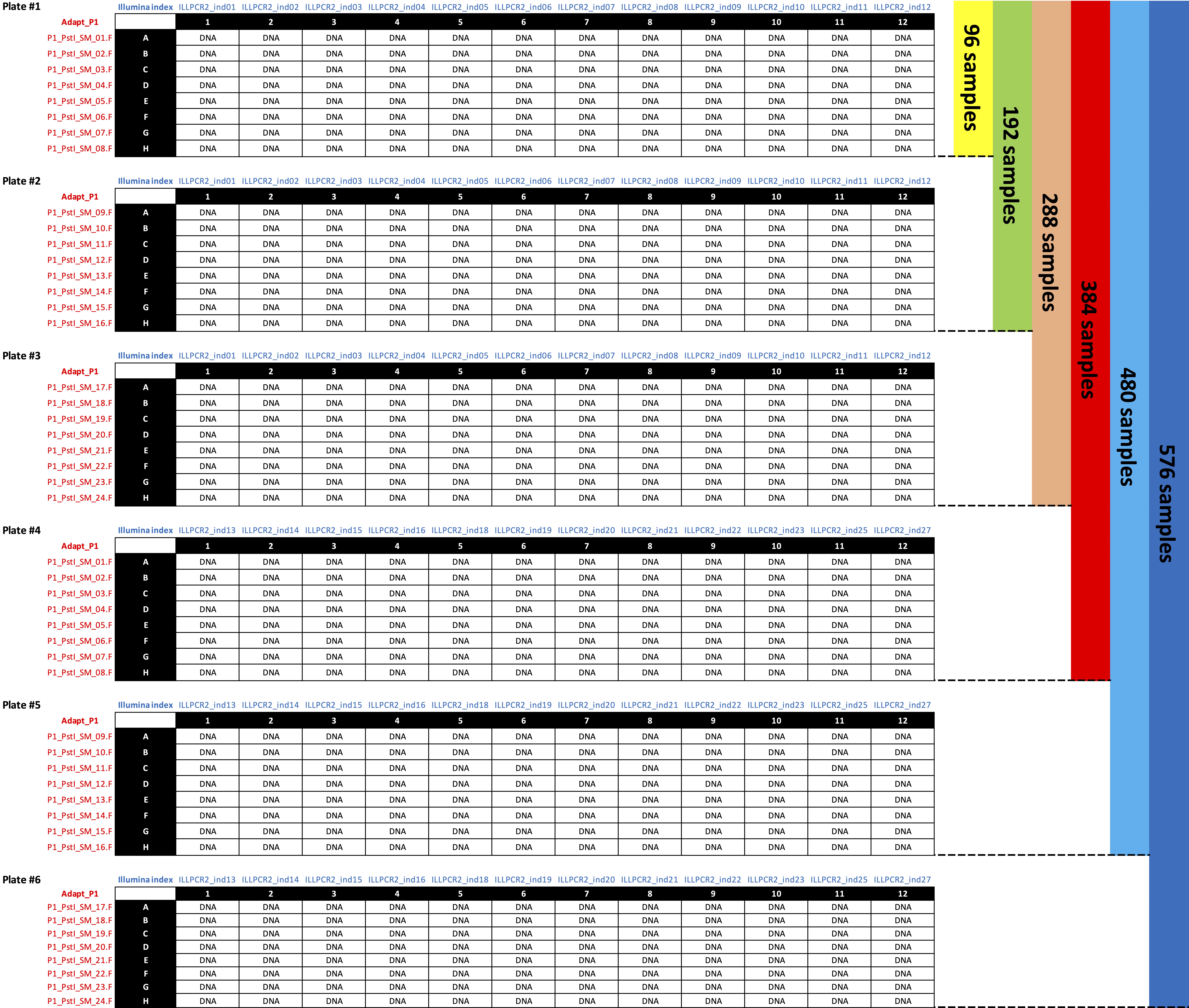
Briefly, purified genomic DNA from the samples are individually digested with 2 restriction enzymes PstI/HhaI PstI/HhaI or PstI/MesI PstI/MesI (one rare-cutter and one more frequent cutter) then ligated to a barcoded adaptor (among 24 available) at one side, and a single adaptor at the other side, purified with magnetic beads, and PCR-amplified allowing the addition of a Illumina index (among 24 available) for multiplexing a maximum of 576 samples per library . Samples are then pooled in equimolar conditions after visualisation on an agarose gel. Purification and size selection is then performed before final quality control of the library and sequencing.
This protocol has proven its effectiveness in several genetic studies of marco-algae populations.



Before start
-
Prepare all buffers and solutions in advance (see Step 1 to Step 5)
-
If not using Retschcopyright Mixer Mill MM 301 (or equivalente) and Grinding ball for the sample grinding, you can use Lysing Matrix H tube with FastPrep-24TM Classic or manual grinding as a last resort.
Steps
Solutions and buffers preparations
5 M sodium chloride solution (NaCl)
29.2g
Dissolve the slat in MilliQ water and fill up to 100mL.
Autoclave.
Store at Room temperature
Annealing buffer stock (10x)
Annealing buffer composed 100 mM Tris-HCl, pH8; 500 mM NaCl and 10 mM EDTA
5mL
5mL
1mL
39mL
Homogenize and autoclave.
Store at Room temperature
Preparation of double-stranded barcoded P1 adaptors 4µM
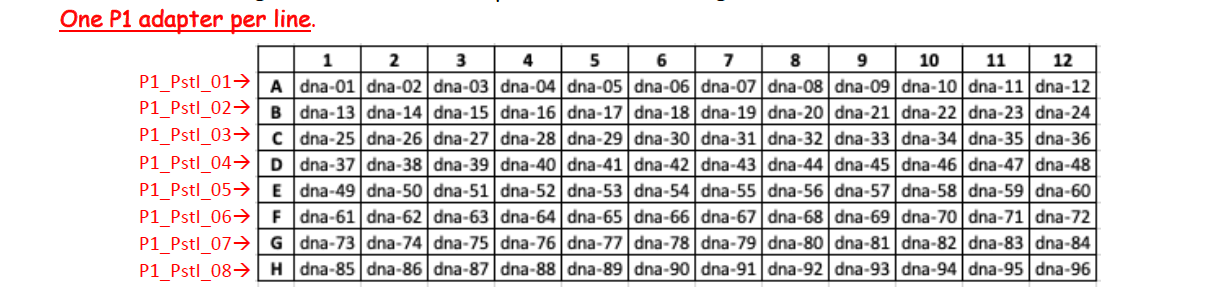
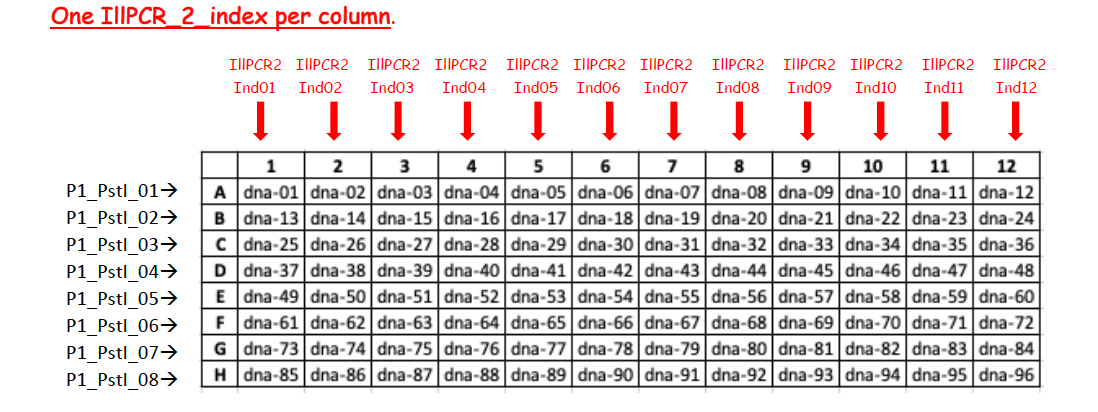
Single-stranded oligos NGS grade P1 need to be annealed with their appropriate partner before ligation. We provide sequences for 48 uniquely barcoded adapter P1 oligo pairs (oligos P1_PstI_x.F and P1_PstI_x.R), see the Barcoded_P1_adaptors.xlsx file below .
To create Adapter P1, combine each oligo Forward with its complementary oligo Reverse in a 1:1 ratio in working strength annealing buffer (final buffer concentration 1x) for a total annealed adapter
concentration of 4µM.
In house barcoded P1 adaptors sequences (NGS grade needed) :
In a PCR plate wells, combine each oligo P1_PstI_x.F with its complementary oligo P1_PstI_x.R :
4µL
4µL
10µL
82µL
| A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|
| P1_PstI_01 | P1_PstI_09 | P1_PstI_17 | |||||
| P1_PstI_02 | P1_PstI_10 | P1_PstI_18 | |||||
| P1_PstI_03 | P1_PstI_11 | P1_PstI_19 | |||||
| P1_PstI_04 | P1_PstI_12 | P1_PstI_20 | |||||
| P1_PstI_05 | P1_PstI_13 | P1_PstI_21 | |||||
| P1_PstI_06 | P1_PstI_14 | P1_PstI_22 | |||||
| P1_PstI_07 | P1_PstI_15 | P1_PstI_23 | |||||
| P1_PstI_08 | P1_PstI_16 | P1_PstI_24 |
Example of a plate map for barcoded P1 adaptors. Allow enough space betweeen the rows to avoid cross-contaminations between barcodes.
The reaction is performed in a thermocycler with the following PCR cycling conditions :
| A | B | C | D |
|---|---|---|---|
| Cycle step | Temperature | Time | Cycles |
| Initial Denaturation | 97.5°C | 2.5 min | 1 |
| Annealing | 96°C (-3°C per cycle) | 1 min | 25 |
| Hold | 4°C |
PCR cycling conditions
Store at4°C (or at-20°C for a long-term storage)
Preparation of double-stranded P2 adaptors 40µM
Single-stranded oligos NGS grade P2 need to be annealed with their appropriate partner before PCR. We provide sequences for 4 uniquely adapter P2 oligo pairs (oligos P2_HhaI.F and P2_HhaI.R or P2_MseI.F and P2_MseI.R), see the No-Barcoded_P2_adaptors.xlsx file below .
To create Adapter P2, combine each oligo Forward with its complementary oligo Reverse in a 1:1 ratio in working strength annealing buffer (final buffer concentration 1x) for a total annealed adapter
concentration of 40µM.
No-barcoded P2 adaptors sequences (NGS grade needed) :
In 1.5mL microtube, combine oligo P2_HhaI.F with its complementary oligo P2_HhaI.F (or P2_MseI.F and P2_MseI.R)
400µL
400µL
100µL
100µL
Then aliquot this volume into 125µL in each well of a 8- PCR tube strip.
The reaction is performed in a thermocycler with the following PCR cycling conditions :
| A | B | C | D |
|---|---|---|---|
| Cycle step | Temperature | Time | Cycles |
| Initial Denaturation | 97.5°C | 2.5 min | 1 |
| Annealing | 96°C (-3°C per cycle) | 1 min | 25 |
| Hold | 4°C |
PCR cycling conditions
Pool all reaction in a1.5mL tube.
Store at 4°C (or at -20°C for a long-term storage).
Preparation of Illumina indexed primers mix (5µM)
In 24 1.5mL microtubes, combine each of the 24 Illumina indexed reverse primers ILLPCR2_ind01 to ILLPCR2_ind27 (no primer numbers ind17, ind24 and ind26) with the Illumina no-indexed forward primer ILLPRC1, see the Illumina_indexed_primers.xlsx file below .
5µL ILLPCR1 oligo forward (100µM)
5µL ILLPCR2 oligo reverse (100µM) ind01 to ind27 (one per tube)
90µL nuclease free water and mix by pipetting
Store at 4°C (or at -20°C for a long-term storage)
Ilumina indexed primers sequences (NGS grade needed) :
Genomic DNA extraction and purification
Genomic DNA extraction
Upon collection, a piece of tissue was cut out from a spot that was free of algal and animal epiphytes and stored in silica gel. Total genomic DNA was extracted from 15 to 20 mg of grinded dry tissue using the Nucleospin 96 plant kit (Macherey-Nagel, Germany).

The extraction was performed according to the manufacturer's instructions using the PL1 lysis buffer except that we added one wash step with PW1 buffer (2 times PW1 washes in total) and one wash step with PW2 buffer (3 times PW2 washes in total). The extracted DNA was eluted into 120 μL (2 x 60 µL) of the supplied elution buffer.
Instruction-NucleoSpin-96-Plant-II.pdf
Store at 4°C (or at -20°C for a long-term storage)
Genomic DNA purification
The genomic DNA extracts were purified using the NucleoSpin gDNA Clean-up XS, Micro kit for DNA clean up and concentration (Macherey-Nagel, Germany).
The purifications were performed according to the manufacturer's instructions with elution into 30 μL (2 x 15 µL) of the supplied elution buffer.
Transferring the 120µL into 1.5 mL microtubes. Add nuclease free water to fill up to 400µL.
The purifications were performed according to the manufacturer's instructions with elution into 30 μL (2 x 15 µL) of the supplied elution buffer.
Removal of residual ethanol and concentration were performed by incubation 0h 15m 0s at 70°C
The purified gDNA of each sample was transferred in a 96 wells PCR plate.
Store at 4°C (or at -20°C for a long-term storage)
Genomic DNA Quality and Quantification
Quantification of Genomic DNA ( Preparation for one 96 wells PCR plate) using PicoGreen‱ TM
Quantify Genomic DNA extract using PicoGreenTM.
Take out all reagents from the fridge and bring them to room temperature.
Take out the DNA samples from the freezer. DNA samples should be slowly thawed on ice
Place the plate in a plate reader and measure the fluorescence according to the following parameters:
Excitation ~480 nm
Emission ~520 nm
Integration time 40 s
Lag time 0 s
Gain Optimal
Number of flashes 10
Calculated well highest standard
Shaking 5 s
Equipment
| Value | Label |
|---|---|
| Synergy 2 | NAME |
| absorbance microplate reader | TYPE |
| BioTek | BRAND |
| Synergy2 | SKU |
Equipment
| Value | Label |
|---|---|
| SPARK | NAME |
| Microwell plate reader | TYPE |
| TECAN | BRAND |
| SPARK | SKU |
Plot the measured fluorescent values of the standard samples against their known concentrations and fit a linear curve using linear regression. Make sure that the coefficient of determination (R2) is close to 1 (typically > 0.99). Calculate the DNA concentrations in the unknown samples using the slope and intercept parameters of the linear equation. Output values you obtained are in pg/µl, assuming 1 µl of each sample was used.
Preparation of of 1X TE buffer 11mL of 1X TE buffer
In 15mL
550µL
10.450mL
Mix by inverting the tube several times.
Preparation of DNA solution at 5000 pg/µl (for 3 ranges)
In 0.5mL
4µL
76µL
Mix by inverting the tube several times.
Preparation of the standard range 0 pg/µl to 1000 pg/µl
Prepare the following standard mixture in 8 0.5mL
| A | B | C | D | E |
|---|---|---|---|---|
| Tubes | Standard DNA solution concentration (pg/µL) | Standard DNA solution volume (µL) | 1X TE (µL) | Final DNA concentration (pg/µL) |
| 1 | 5000 | 42 | 168 | 1000 |
| 2 | 5000 | 21 | 189 | 500 |
| 3 | 5000 | 9 | 171 | 250 |
| 4 | 1000 | 21 | 189 | 100 |
| 5 | 500 | 21 | 189 | 50 |
| 6 | 100 | 18 | 162 | 10 |
| 7 | 50 | 18 | 162 | 5 |
| 8 | 0 | 0 | 180 | 0 |
Standard DNA solutions preparation
Pipette 50µL of each standard mixture in the first two columns of the black, sterile, 96-well plate :
| A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|
| 1000 pg/µL | 1000 pg/µL | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA |
| 500 pg/µL | 500 pg/µL | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA |
| 250 pg/µL | 250 pg/µL | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA |
| 100 pg/µL | 100 pg/µL | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA |
| 50 pg/µL | 50 pg/µL | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA |
| 10 pg/µL | 10 pg/µL | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA |
| 5 pg/µL | 5 pg/µL | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA |
| 0 pg/µL | 0 pg/µL | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA | unknown DNA |
Exemple of map plate for PicoGreen‱ quantification
Pipette 49 µl of 1X TE buffer in the remaining wells.
49µL
Pipette 1 µl of the unknown DNA samples in the remaining wells.
1µL
Prepare PicoGreen‱ work solution ® work solution
In 10mL
25µL
4.975mL
Mix and protect from light.
Pipette 50µL in each well, including the standard and unknown sample wells.
Protect the 96-well plate from light and incubate for 0h 5m 0s at room temperature.
Genomic DNA preparation
In a PCR plate, put around 100ngof genomic DNA in a volume of 40µL (in nuclease free water or Tris-HCl 5mM pH 8.5) for each sample. If possible, randomize the location of samples in the microplate. Keep a few empty wells for negative controls.
Double digestion
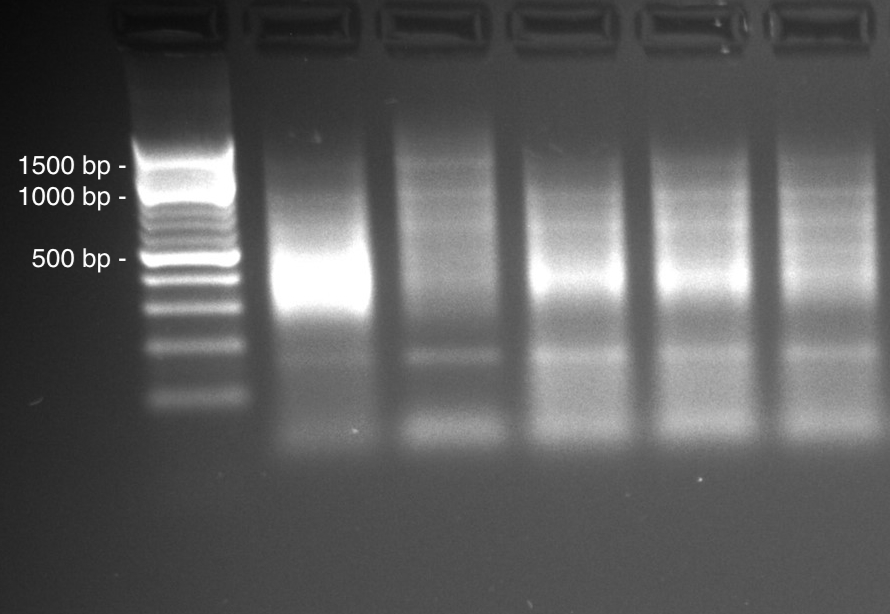
Double digest around 100ng of high quality genomic DNA with selected restriction enzymes 50µLreaction volume. Use a digestion buffer appropriate for both enzymes. Here, we will do the protocol for the PstI and HhaI couple of enzymes but it's same with PstI and MseI couple. Both couple of enzyme woks well for micro-algae but it's possible to test double digestion on few sample to select the best couple of enzymes. The best couple given large smear with size range 100 bp to 1000 pb.
Vortex all reagents, except enzymes (stored at -20°C), for approximately 0h 0m 5s
Spin down all reagents for approximately 0h 0m 5s and placeOn ice.
In a microtube, prepare the digestion mix, according to the following table for a total volume of 50µL:
| A | B | C | D | E |
|---|---|---|---|---|
| Initial concentration | Final concentration | n=1 | n=100 (1 plate) | |
| Genomic DNA | ~100 ng | 40 µL | ||
| Cutsmart buffer | 10X | 1X | 5 µL | 500 µL |
| Enzyme 1 (PstI HF) | 20 u/µL | 10U | 0.5 µL | 50 µL |
| Enzyme 2 (HhaI or MseI) | 20 u/µL | 10U | 0.5 µL | 50 µL |
| nuclease-free water | 4 µL | 400 µL | ||
| TOTAL | 50 µL | 1000 µL |
Digestion master mix composition
Vortex the master mix and spin down.
Aliquot 125µL of the digestion master mix in each well of a 8-PCR tube strip.
In the DNA plate (containing 40µL per well), add10µL of digestion master mix with a x8 multichannel pipette and mix by pipetting, seal PCR plate and spin down.
Incubate at 37°C 0h 0m 5s
Then store at 4°C
Check digestion on an agarose gel
Bead purification (96‑well plate format)
This protocol can be used to remove contaminants, unligated adapters, enzymes, buffer additives, salts... and short DNA fragments. The method utilizes a single-size selection step : After adding the appropriate volume of Bead Suspension to the DNA sample, beads will bind larger fragments. The supernatant contains smaller fragments and contaminants that are discarded. For most NGS sequencing applications it is optimal to remove all fragments below 100 bp. This can be achieved by using a volume ratio (bead suspension to sample) of 1:1, which is described in the following protocol.
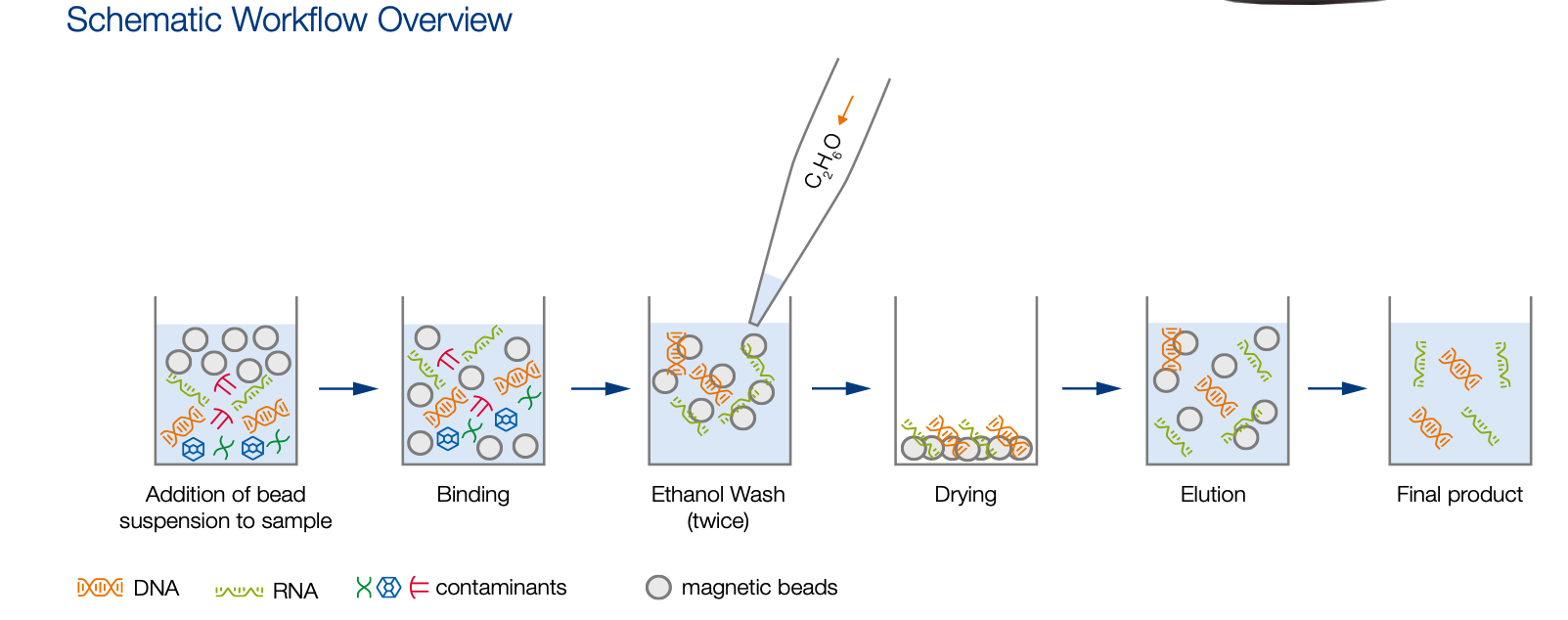
Before starting
Prepare 50mL of fresh 80% Molecular Biology Grade Ethanol
40mL
10mL
Remove the NucleoMag® NGS Bead Suspension from the fridge. Let for approximately 30 min to bring the bead suspension to Room temperature.
Then, vortex this Bead Suspension stock solution carefully until homogenized and put in a reagent reservoir.
Binding
This step binds DNA fragments 100 bp and larger to the magnetic beads.
Pipette 45µL of NGS Beads suspension with x8 multichannel pipette and transfer in digestion plate (plate with 45µL of digested template DNA for each sample), carefully mix by pipetting up and down 10 times.
Incubate 0h 5m 0s atRoom temperature
Separation
Place the purification plate onto the 96-well magnetic separator.
Wait at least 0h 5m 0s until all the beads have been attracted by the magnets or until the liquid appears clear.
The supernatant contains unwanted low molecular weight contaminants and unwanted smaller DNA fragments.
Remove and discard the supernatant (~90 µl) by pipetting.
1st wash with 80 % ethanol
Place 80% ethanol in a reagent reservoir.
With a x8 multichannel pipette, dispense200µL of 80% ethanol into the purification plate without disturbing the bead pellet.
Incubate the purification plate at room temperature for at least 0h 0m 30s
Carefully and completely remove and discard ethanol by pipetting.
2nd wash with 80 % ethanol
With a x8 multichannel pipette, dispense200µL of 80% ethanol into the purification plate without disturbing the bead pellet.
Incubate the purification plate at room temperature for at least 0h 0m 30s
Carefully and completely remove and discard ethanol by pipetting.
Dry the beads
Let the purification plate on the magnetic separator and incubate at Room temperature for maximum 0h 5m 0s in order to allow the remaining traces of ethanol to evaporate.
Elute DNA fragments
Take the purification plate from the magnetic stand, and add 40µL of nuclease-free water with a x8 multichannel pipette to resuspend the bead pellet by pipetting up and down 10 times.
Incubate the purification plate at Room temperature for 0h 5m 0s .
Separate the magnetic beads against the side of the wells by placing the 96-well plate on the magnetic separator.
Wait at least 0h 5m 0s until all the beads have been attracted by the magnets or until the liquid appears clear.
Transfer 35µL of the supernatant containing the digested purified template DNA to a new 96‑well plate. Be careful to avoid pipeting beads during this step .
Seal the plate and store at 4°C (or store -20°C for a long-term storage) until adaptor ligation.
Adaptor ligation
For each sample of one line of the digested purified plate (with 35µL of digested purified template DNA) add 5µL of double-stranded barcoded P1 adaptors at 4 µM. Use one double-stranded barcoded P1 adaptors per line .
Vortex all reagents, except enzymes (stored at -20°C), for approximately 0h 0m 5s
Spin down all reagents for approximately 0h 0m 5s and placeOn ice.
In a microtube, prepare the ligation mix, according to the following table for a total volume of 60µL:
| A | B | C | D | E |
|---|---|---|---|---|
| Initial concentration | Final concentration | n=1 | n=100 (1 plate) | |
| Digested purified template DNA + P1 adaptor | 40 µL | |||
| P2 adaptor (HhaI or MseI) | 40 µM | 330 nM | 0.5 µL | 50 µL |
| T4 ligase buffer | 10X | 1X | 6 µL | 600 µL |
| T4 ligase | 400 u/µL | 160U | 0.4 µL | 40 µL |
| nuclease-free water | 13.1 µL | 1310 µL | ||
| TOTAL | 60 µL | 2000 µL |
Ligation master mix composition
Vortex the master mix and spin down.
Aliquot 125µL of the ligation master mix in each well of two 8-PCR tube strip.
In the digested purified plate (containing 35µL of digested purified template DNA and 5µLof barcoded P1 adaptors ), add20µL of ligation master mix with a x8 multichannel pipette and mix by pipetting, seal PCR plate and spin down.
Incubate at 16°C 0h 0m 5s
Then store at 4°C or at -20°C if not performing the bead purification the day after.
Before starting
Prepare 50mL of fresh 80% Molecular Biology Grade Ethanol
40mL
10mL
Remove the NucleoMag® NGS Bead Suspension from the fridge. Let for approximately 30 min to bring the bead suspension to Room temperature.
Then, vortex this Bead Suspension stock solution carefully until homogenized and put in a reagent reservoir.
Binding
This step binds DNA fragments 100 bp and larger to the magnetic beads.
Pipette 60µL of NGS Beads suspension with x8 multichannel pipette and transfer in adaptor-ligated plate (plate with 60µL of digested and adaptor-ligated template DNA for each sample), carefully mix by pipetting up and down 10 times.
Incubate 0h 5m 0s atRoom temperature
Separation
Place the purification plate onto the 96-well magnetic separator.
Wait at least 0h 5m 0s until all the beads have been attracted by the magnets or until the liquid appears clear.
The supernatant contains unwanted low molecular weight contaminants and unwanted smaller DNA fragments.
Remove and discard the supernatant (~120 µl) by pipetting.

1st wash with 80 % ethanol
Place 80% ethanol in a reagent reservoir.
With a x8 multichannel pipette, dispense200µL of 80% ethanol into the purification plate without disturbing the bead pellet.
Incubate the purification plate at room temperature for at least 0h 0m 30s
Carefully and completely remove and discard ethanol by pipetting.
2nd wash with 80 % ethanol
With a x8 multichannel pipette, dispense200µL of 80% ethanol into the purification plate without disturbing the bead pellet.
Incubate the purification plate at room temperature for at least 0h 0m 30s
Carefully and completely remove and discard ethanol by pipetting.
Dry the beads
Let the purification plate on the magnetic separator and incubate at Room temperature for maximum 0h 5m 0s in order to allow the remaining traces of ethanol to evaporate.
Elute DNA fragments
Take the purification plate from the magnetic stand, and add 40µL of nuclease-free water with a x8 multichannel pipette to resuspend the bead pellet by pipetting up and down 10 times.
Incubate the purification plate at Room temperature for 0h 5m 0s .
Separate the magnetic beads against the side of the wells by placing the 96-well plate on the magnetic separator.
Wait at least 0h 5m 0s until all the beads have been attracted by the magnets or until the liquid appears clear.
Transfer 35µL of the supernatant containing the adaptor-ligated purified template DNA to a new 96‑well plate. Be careful to avoid pipeting beads during this step .
Seal the plate and store at 4°C (or store -20°C for a long-term storage) until PCR amplification.
Rad Tag enrichment (PCR)
PCR amplification to generate Illumina sequencing indexed libraries :
In this PCR, Illumina indexed primers are incorporated in order to produce fragments compatible with Illumina sequencing, and to insert an index allowing multiplexing of barcoded samples. This index will be read during the sequencing run.
This PCR is expected to have a homogenizing effect. Primers are thus included in limiting quantity, in order to produce equalized amounts of PCR fragments among samples. The number of cycles is limited to a maximum of 15 (optimal with 12). After those cycles, a final PCR cycle is then performed after addition of primers in large excess.
The Reaction mixture for a total volume of 40µL is :
| A | B | C | D |
|---|---|---|---|
| Initial concentration | Final concentration | n=1 | |
| Adaptor-ligated purified template DNA | 10 µL | ||
| Primer mix (ILLPCR1 and ILLPCR2ind) | 5 µM each | 0.17 µM | 1.36 µL |
| Q5 buffer | 5X | 1X | 8 µL |
| High GC enhancer | 5X | 1X | 8 µL |
| dNTP mix | 25mM each | 0.20 µM | 0.32 µL |
| Q5 hotstart hifi polymerase | 2 u/µL | 0.8 U | 0.40 µL |
| nuclease-free water | 11.92 µL | ||
| Total mix | 30 µL | ||
| TOTAL reaction | 40 µL |
PCR mixture composition
We need to prepare one PCR mixture per index, i.e. 12 PCR mixtures for one plate.
First PCR mix preparation (with primers in limiting quantity)
Defreeze and vortex all reagents, except enzymes (stored at -20°C), for approximately0h 0m 5s
Spin down all reagents for approximately0h 0m 5s and place On ice.
In 12 0.5mL microtubes, prepare the 1st mix according to the following table (one mix per column) :
| A | B | C | D | E |
|---|---|---|---|---|
| Initial concentration | Final concentration | n=1 | n=10 (one column of 1 plate) | |
| Adaptor-ligated purified template DNA | 10 µL | |||
| Primer mix (ILLPCR1 and ILLPCR2ind) | 5 µM each | 0.17 µM | 1.36 µL | 13.6 µL |
| Q5 buffer | 5X | 1X | 8 µL | 80 µL |
| High GC enhancer | 5X | 1X | 8 µL | 80 µL |
| dNTP mix | 25mM each | 0.20 µM | 0.32 µL | 3.2 µL |
| Q5 hotstart hifi polymerase | 2 u/µL | 0.8 U | 0.40 µL | 4 µL |
| nuclease-free water | 11.92 µL | 119.2 µL | ||
| TOTAL | 30 µL | 300 µL |
PCR mix composition
Vortex mix all reagents in the mix and spin down.
In a new PCR plate, dispense 30µL of 1st mix in each column.
DNA and mix combination
Spin down the adaptor-ligated purified template DNA plate.
With a multichannel pipette, transfer 10µL of adaptor-ligated purified template DNA into the PCR plate and mix by pipetting.
Finally, aliquot the 40µL of the total mix by dispensing 20µL into 1 additional new empty PCR plates.
Seal the 2 PCR plates and spin down.
The 2 PCR will be performed in parallel in 2 different thermal cyclers, in order to reduce the PCR bias.
| A | B | C | D |
|---|---|---|---|
| Cycle step | Temperature | Time | Cycles |
| Hot start initial denaturation | 98°C | 30 sec | 1 |
| Denaturation | 98°C | 20 sec | 15 |
| Annealing | 60°C | 30 sec | 15 |
| Extension | 72°C | 40 sec | 15 |
| Final extension | 72°C | 10 min | 1 |
| Hold | 4°C |
PCR program for the Illumina indexing PCR
After PCR, pool back the 2 PCR plates into a single plate with a multichannel
Final cycle (with primers in large excess)
In a 12-tube PCR strip, prepare the 2nd mix according to the following table (one mix per column) :
| A | B | C | D | E |
|---|---|---|---|---|
| Initial concentration | Final concentration | n=1 | n=10 (one colomn of 1 plate) | |
| Primer mix (ILLPCR1 and ILLPCR2ind) | 5 µM each | 3.35 µM | 2.68 µL | 26.8 µL |
| Q5 buffer | 5X | 1X | 0.80 µL | 8 µL |
| dNTP mix | 25mM each | 0.20 µM | 0.32 µL | 3.2 µL |
| nuclease-free water | 0.20 µL | 2 µL | ||
| TOTAL | 4 µL | 40 µL |
Final cycle PCR mix composition
Mix all reagents by pipetting and spin down.
Dispense 4µL of final cycle mix in each line of the PCR plate with a 12 multichannel pipette.
Seal the PCR plate and spin down.
In a thermocycler, run the final cycle as follows :
| A | B | C | D |
|---|---|---|---|
| Cycle step | Temperature | Time | Cycles |
| Denaturing | 98°C | 3 min | 1 |
| Annealing | 60°C | 2 min | 1 |
| Extension | 72°C | 12 min | 1 |
| Hold | 12°C |
PCR program for the final cycle of the illumina PCR
After PCR, place the plate at4°C (or -20°C for a long-term storage).
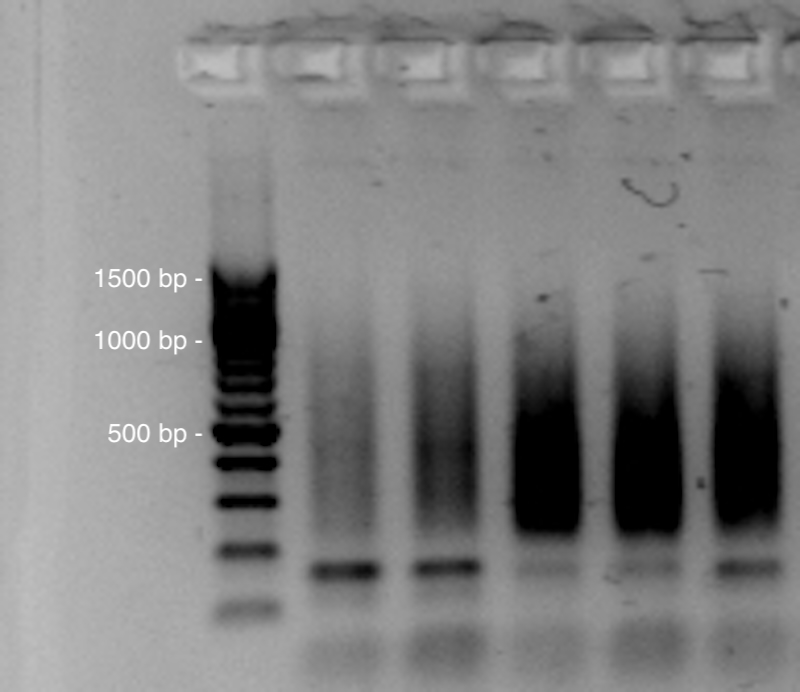
Check PCR on an agarose gel
Sample pooling (equimolar)
Each barcoded and indexed individual can now be pooled in a single tube, in equimolar conditions.
After the normalizing PCR, all smears should have similar intensity on the agarose gel. In this case, pool 5µL of all individuals in a single low binding 1.5mL microtube.
If not, normalization can be made at this step. For this, roughly estimate the concentration of fragments from the gel picture, and pools accordingly. It can be efficient to make intermediate pools for example, one pool for the low, one for the medium, and another one for the high intensity samples in 3 low binding 1.5mLmicrotubes .
Vortex mix and spin down.
Store at4°C (or -20°C for a long-term storage) until bead purification.
Bead purification (microtube format)
Before starting
Prepare 10mLof fresh 80% Molecular Biology Grade Ethanol
8mL
2mL
Remove the NucleoMag® NGS Bead Suspension from the fridge. Let for approximately 30 min to bring the bead suspension to Room temperature.
Then, vortex this Bead Suspension stock solution carefully until homogenized and put in a reagent reservoir.
Binding
This step binds DNA fragments 100 bp and larger to the magnetic beads.
Pipette a volume of NGS Beads suspension to have a ratio 1:1 with the sample pooling volume, and transfer in the pooling sample tube(s).
Carefully mix by pipetting up and down 10 times.
Incubate 0h 5m 0s atRoom temperature
Separation
Place the purification tube(s) onto the magnetic microtube stand.
Wait at least 0h 5m 0s until all the beads have been attracted by the magnets or until the liquid appears clear.
The supernatant contains unwanted low molecular weight contaminants and unwanted smaller DNA fragments.
Remove and discard the supernatant by pipetting.
1st wash with 80 % ethanol
Dispense1mL of 80% ethanol into the purification tube(s) without disturbing the bead pellet.
Incubate the purification tube(s) at room temperature for at least 0h 0m 30s
Carefully and completely remove and discard ethanol by pipetting.
2nd wash with 80 % ethanol
Dispense1mL of 80% ethanol into the purification tube(s) without disturbing the bead pellet.
Incubate the purification tube(s) at room temperature for at least 0h 0m 30s
Carefully and completely remove and discard ethanol by pipetting.
Dry the beads
Let the purification tube(s) on the magnetic separator and incubate at Room temperature for maximum 0h 5m 0s in order to allow the remaining traces of ethanol to evaporate.
Elute DNA fragments
Take the purification tube(s) from the magnetic stand, and add 100µL of nuclease-free water with a pipette to resuspend the bead pellet by pipetting up and down 10 times.
Incubate the purification tube(s) at Room temperature for 0h 5m 0s .
Separate the magnetic beads against the side of the tube by placing the tube(s) on the magnetic separator.
Wait at least 0h 5m 0s until all the beads have been attracted by the magnets or until the liquid appears clear.
Transfer 90µL of the supernatant in a new(s) low binding 1.5mL microtube(s). Be careful to avoid pipeting beads during this step .
Store at 4°C (or store -20°C for a long-term storage) until size selection.
Intermediate pools quantification and final pooling (Optional)
If you made intermediate pools (low, medium and high intensity on agarose gel), estimate the double strand DNA concentration in the pools by fluorimetry with a Qubit equipment.
Pool in equimolar concentration the 3 intermediate pools in a single low binding 1.5mLmicrotube with a minimum concentration of 20nanomolar (nM) and minimum volume of 30µL.
Vortex mix and spin down.
Store at4°C (or -20°C for a long-term storage) until size selection.
Size selection with sage science Pippin-Prep
Perform the size selection of fragments between 300 and 800 bp using a 1,5% DF marker K agarose gel cassette, according to the Pippin prep manufacturer's instructions :
Pippin-prep-Quick-Guide-CDF1510-marker-K3.pdf
Quality control of the libraries
Control the quality of the library with a Bioanalyzer (Agilent) (or equivalent equipment) in a High Sensitivity DNA chip. Dilute your pool 1:2 or more and load 1µl of the pool before and after size selection, according to the manufacturer's instructions :
Agilent_high_SensitivityDNA_KG_EN.pdf
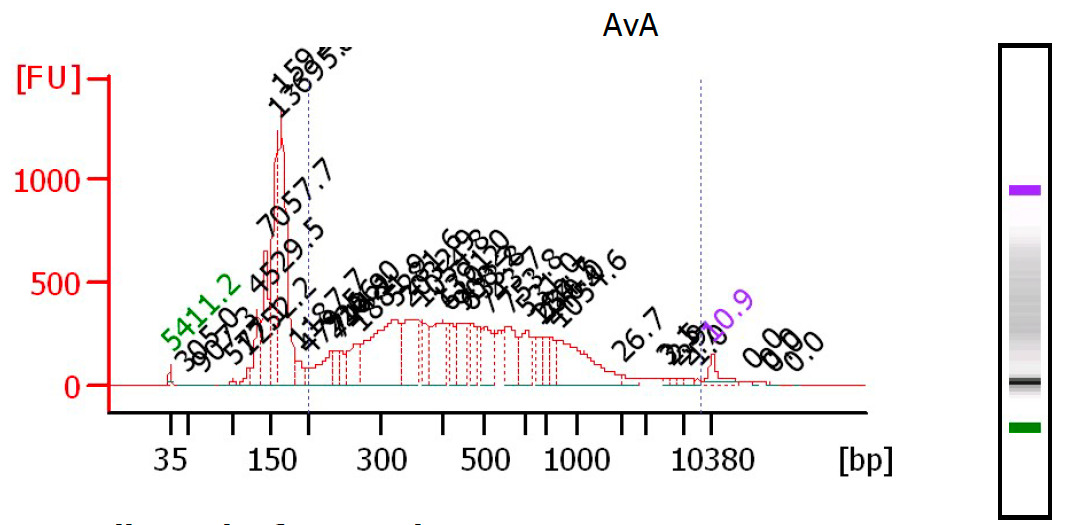
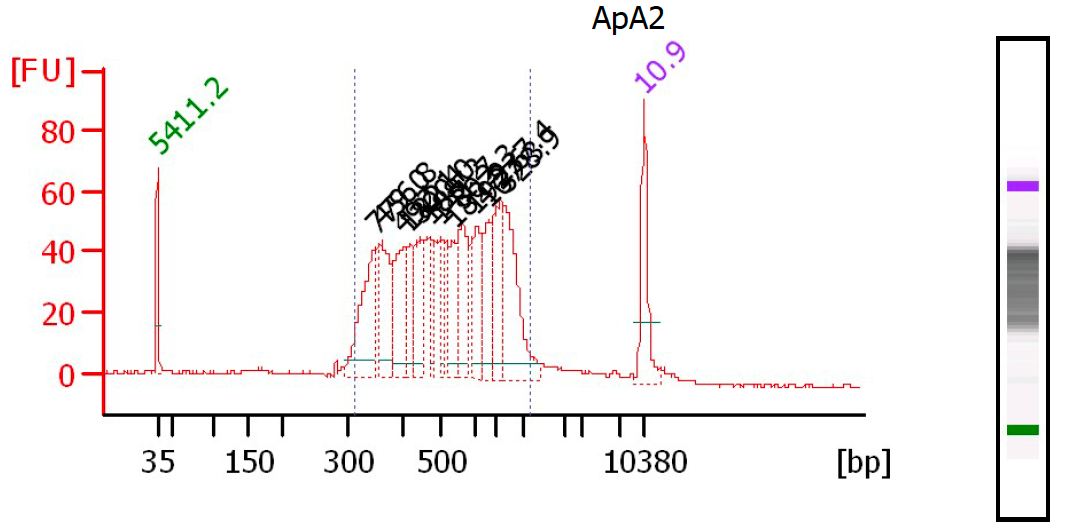
Fluorimetric estimation of the dsDNA concentration in the library.
Perform a quick estimation of the DNA concentration with a fluorimetric assay, in a QubitTM apparatus or equivalent, with the Qubit ds 1X DNA HS assay kit, according to the manufacturer's instructions:
qPCR quantification
In the case you need an accurate estimation of the DNA concentration in your library, perform a qPCR quantification with the NEBNext Library Quant Kit for Illumina, or equivalent, which uses P5 and P7 illumina primers to target the double stranded DNA fragments in the library. Follows the kit's user guide and perform your quantitative qPCR in a qPCR thermocycler (e.g. LightCycler 480, Roche).
Contrarily to the fluorimetric method (Qubit), the qPCR estimation will only consider dsDNA fragments starting with P5 and ending with P7 illumina sequences, that will be effectively amplified onto the flowcell of the Illumina sequencer.
Suggestions to prepare library dilutions for qPCR
In 0.5mL low binding microtube, prepare 1:1 000 dilution of library with buffer supplied in the qPCR kit. Then, prepare the 3 library dilutions (1:10 000 to 1:30 000) to be used on triplicate for qPCR analysis.
1:100 : 1µL of library + 99µL of 1X buffer
1:1 000 : 10µL of library + 90µL of 1X buffer
1:10 000 : 20µL of 1:1 000 dilution + 180µL of 1X buffer
1:20 000 : 50µL of 1:10 000 dilution + 50µL of 1X buffer
1:30 000 : 50µL of 1:10 000 dilution + 100µL of 1X buffer
You should get more than 10 nM, that is the library concentration usually required by the sequencing platform facilities.
Use the average size of the library size range as estimated from the Bioanalyzer profile to convert DNA concentration from nM to ng/µL using the attach file below :
Library ready for sequencing
The library is now ready for sequencing in single read or paired-end 150 bases in an Illumina sequencer, with one index read.