Chemiluminescence-enhanced ELISA measurements for α-Synuclein fibrils
Arpine Sokratian
Disclaimer
Optimized biolegend LEGEND MAXTM α-Synuclein Aggregate ELISA Kit
Abstract
This protocol is optimized The BioLegend LEGEND MAXTM α-Synuclein Aggregate ELISA Kit in a 96-well strip plate precoated with rat monoclonal anti-α-synuclein aggregate antibody. We use in-house a-syn fibrils and monomeric protein as a control. We use ELISA to detect a-syn fibril levels in neuronal lysates after 14 days of fibril-treatment.
Steps
Preparation of buffers
Preparation of 1X Reagent Diluent
-
Label an appropriate sized bottle as “1X Reagent Diluent”.
-
Dilute 2X Reagent Diluent to 1X by adding 30mL of 2X Reagent Diluent to 30mL of lab grade water in the bottle labeled “1XReagentDiluent”.
-
Mix well by vortex.
Preparation of 1X Wash Buffer
-
Label a 1L bottle as “1X Wash Buffer”.
-
Dilute 5X Wash Buffer 1:5 using lab grade water* and mix well.
Preparation of standard intermediates
Thaw down On ice monomer/sonicated fibril preparation aliquots stored at -80°C
Measure monomer concentration via Nanodrop
Add Amount of 5x, 10x or 20x diluted aliquots in PBS onto nanodrop piedestal;
Parameters: other proteins; coefficient extinction:
5.98; MW: 14.4 kDA for human a-synuclein
7.45; MW: 14.4 kDA for mouse a-synuclein
Perform two measurements for each dilution and confirm <10% standard error between two measurements
For fibril preparations, dilute in 6M guanidine HCL and follow the aforementioned instructions derscribed for monomeric protein
Equipment
| Value | Label |
|---|---|
| NanoDrop™ One/OneC Microvolume UV-Vis Spectrophotometer | NAME |
| UV-Vis Spectrophotometer | TYPE |
| Thermo Scientific | BRAND |
| ND-ONE-W | SKU |
| https://www.thermofisher.com/us/en/home.html | LINK |
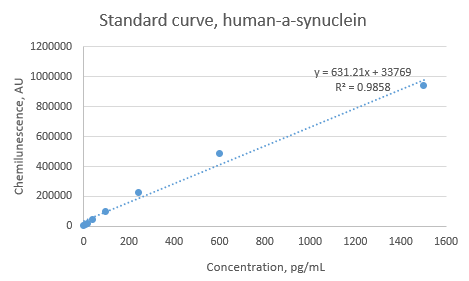
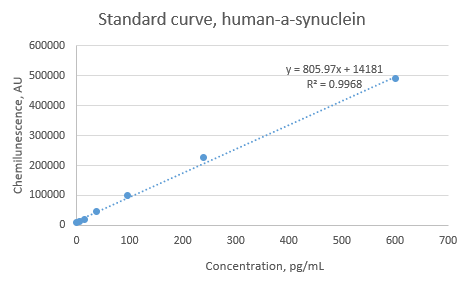
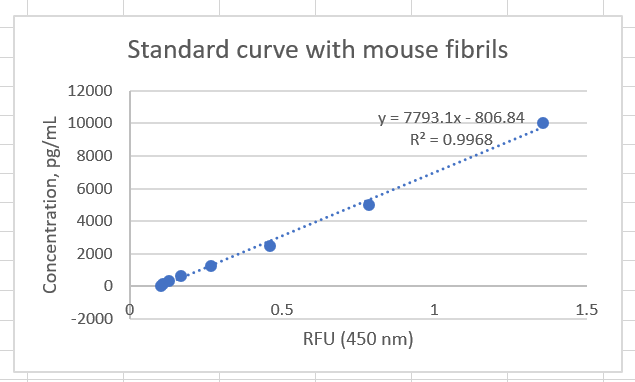
Use 1X Reagent Diluent to prepare the standard intermediates as follows:
Assay reaction 0 : dilute monomer stock to a concentration 50ng/mL 50ng/mL
Assay reaction // Volume of standard (uL) // Volume of 1X reagent diluent (uL) // Final concentration (pg/mL)
1 40 uL of \#0 1280 1500
2 550 uL of \#1 825 600
3 550 uL of \#2 825 240
4 550 uL of \#3 825 96.0
5 550 uL of \#4 825 38.4
6 550 uL of \#5 825 15.4
7 550 uL of \#6 825 6.1
8 0 825 0
Sample preparation
Dilute samples in 1X Reagent Diluent; if correct dilution is unknown - prepare a standard dilutions as follows: 10X; 20X, 40x;100X; 500X;
Once evaluated - use correct dilution factor to run an experiment;
Minimum recommended dilution is 1:10 ;
we recommend dilution for neuronal lysates to be set at 1:100
ELISA procedure
Remove the plate from the foil pouch.
Add 300µL of 1X Wash Buffer (use multichannel pipette and ELISA racks to easily access the wash buffer).
Dump out wash buffer and pat dry.
Repeat 3 more times for a total of 4 washes.
Add 50 μL of 1X Reagent Diluent to each well that will contain either standard dilutions or samples.
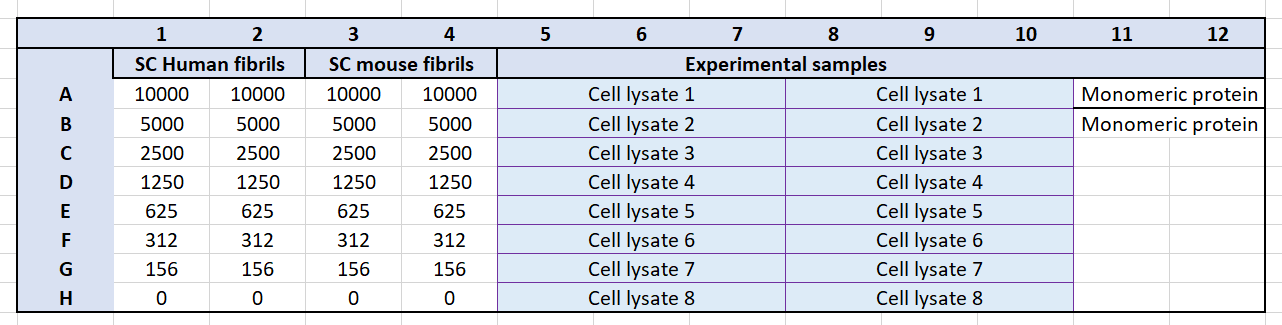
Add 50µL of each standard to the plate in duplicate or triplicate. Follow the plate layout below.
Note: The volumes in Table 2 are sufficient for running the standard curve in triplicate.
Add 50µL of each sample dilution to the plate.
Add 100µL μL of α-Synuclein Aggregate Detection Antibody to each well, seal the plate, and incubate at room temperature for 1 hour with shaking.
Dump the content of the plate and repeat
Dump the content and add Avidin-HRP 100µL to each used well, cover and incubate for 0h 30m 0s at Room temperature
Remove plate from incubation and dump components; repeat
Discard the contents of the plate into a sink, then wash the plate 5 times with 1X Wash Buffer as in step 4. For this final wash, soak wells in 1X Wash Buffer for 30 seconds to 1 minute for each wash. This will help minimize background.
Add 100µL of Substrate Solution F to each well and incubate at room temperature for 15 minutes in the dark.* Wells containing α-synuclein aggregates should turn blue in color with an intensity proportional to its concentration. It is not necessary to seal the plate during this step.
Stop the reaction by adding 100 μL of Stop Solution to each well. The
solution color should change from blue to yellow.
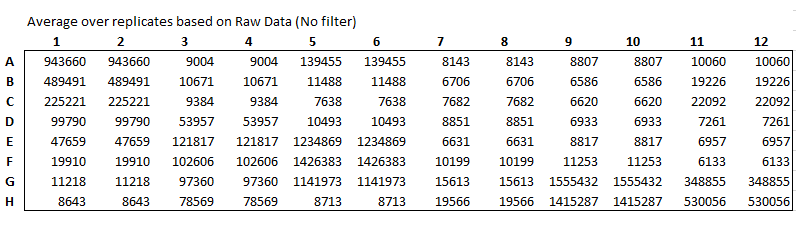
Read plate immediately using ClarioStar plate reader
Equipment
| Value | Label |
|---|---|
| Microplate Reader | NAME |
| BMG Labtech CLARIOstar | BRAND |
| None | SKU |
Basic settings
Measurement type: Luminescence
Microplate name: SBS STANDARD 96
Endpoint settings
Measurement interval time [s]:0.35
Scan mode: orbital averaging
Scan diameter [mm]:4
Optic settings
Emission: No filter
Gain:2000
Well(s) used for gain adjustment:A1
Focal height [mm]:12
General settings
Top optic used
To allow comparison of measurements with different measurement interval times values are normalized to 0.5 sec.
Aperture spoon:-
Injection needle holder type:-
Reading direction: bidirectional, horizontal left to right, top to bottom
Target temperature [°C]:set off
Target concentration O2 [%]:set off
Target concentration CO2 [%]:set off