Bacterial-induced neutrophilic nasal inflammation in mice
Alba Sánchez Montalvo, Aaron Ziani Zeryouh, Marylène Lecocq, Charles Pilette, Valérie Hox
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer-reviewed and may not have undergone formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
The present surgical procedure provides a step-by-step and detailed protocol with all the critical information to develop a bacterial-induced neutrophilic local inflammation in the nasal mucosa of mice. The protocol is a modified and optimized version of the work previously published by Jacob et al. in 2001. The procedure consists of exposing and drilling out the nasal bones and the upper part of the septum followed by the insertion of a bacterial-inoculated nasal tampon in the nasal cavity which remains in situ until sacrifice.
Steps
The day before surgery
Prepare the chosen bacteria
Culture the corresponding bacteria on sheep blood agar plates (BD, Biosciences 254071), according to their specific growth requirements.
In our hands, bacteria strains used were Pseudomonas aeruginosa VitroidsTM (Sigma-Aldrich, VT000256), Staphylococcus aureus LenticulesTM (Sigma-Aldrich, CRM06571M) and Streptococcus pneumoniae (ATCC®, 49619TM). Overnight culture conditions were 37°C for S. aureus and P. aeruginosa and 37°C in 5% CO2 for S. pneumoniae.
Prepare the nasal tampon
In our hands, a Merocel pope ear wick (Medtronic, MI, USA) was cut at a dimension of 4 mm x 1 mm x 1 mm and sterilized by dry heat 100ºC during 3h.
Pre-surgery
Prepare and sterilize your instruments for the surgical procedure
Stereomicroscope (Motic SMZ-171)
1.5-mm microdrill (Medtronic, MI, USA)
Sterile fields (Hartmann Mediset®)
Temperature monitor (PhysioSuite® Kent Scientific Corporation)
15-mm scalpel blade
5.0 non-resorbable polypropylene sutures (MonosoftTM, Covidien)
Scalpel
Forceps
Scissors
Needles
Gloves (CardinalHealthTM ProtexisTMPI micro surgical gloves)
Eye drops (Ocry-gel, TVM)
Chlorhexidine digluconate (Hibidil®, Regent Medical).
Tape
IR lamp (IR100 Infrared lamp, MEDISANA®)
Ultrasonic bath (Clifton)
Surgery platform
Prepare your bacterial solution
From the bacterial cultures, take isolated colonies and prepare serial dilutions up to 109 CFU/ml in NaCl 0.9% and measure it with a spectrophotometer with a reference optical absorbance at 600nm (OD600nm) = 1.
Administration of anesthesia and analgesia to the mouse
Weight the mouse and administer general anesthesia accordingly: a mix of Xylazin (maximum 15mg/kg) and Ketamin (maximum 80 mg/kg) intraperitoneally.
Administer analgesia: Temgésic (maximum 0,05 mg/kg) subcutneously.
Wait 15-20 minutes for the aesthesia and analgesia to do their effect before starting the surgical intervention.
Surgical procedure
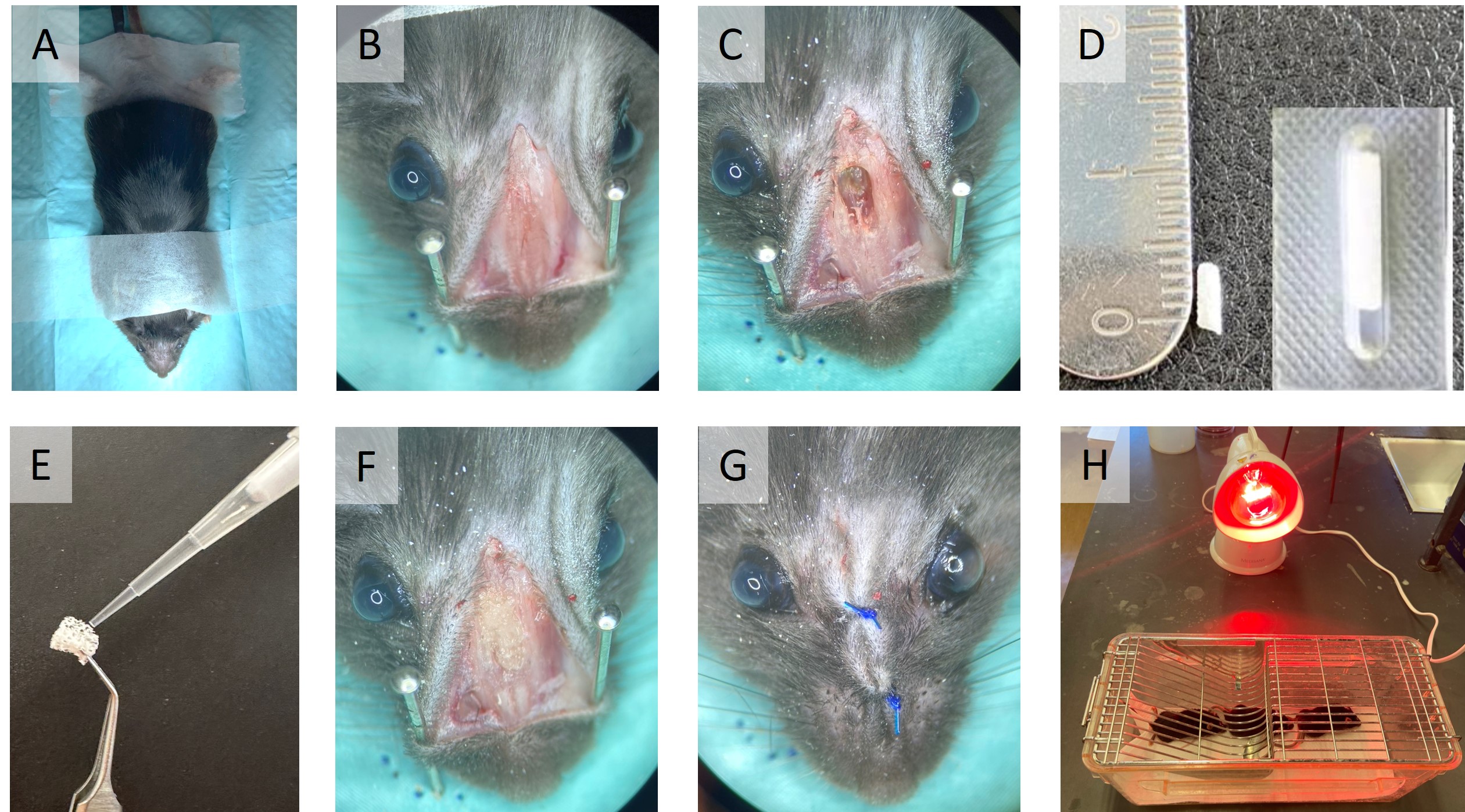
Shave the snout over the intervention area and fix the mouse on a sterile field (Figure 1, A).
Monitor and maintain the intraoperative body temperature of the mouse during the procedure.
Disinfect the intervention area with Chlorhexidine digluconate and use eye drops to prevent eyes from drying.
Use a 15-mm scalpel blade to make a 8-mm midline incision over the nasal dorsum to the snout. Raise skin flaps laterally to expose the nasal bone (Figure 1, B).
Use a 1.5-mm microdrill to drill the nasal bone over the nasal fossa and remove the upper part of the septum. Be extremely careful not to drill completely the bucco-sinusal bone communication. Clear out any bleeding to prevent aspiration (Figure 1, C).
Inoculate the pre-cut nasal tampon with 10 μL of the corresponding bacterial solution or saline for controls and carefully place it into the nasal cavity (Figure 1, D-F).
Suture skin flaps with 5.0 non-resorbable polypropylene sutures and dettach the mouse from the surgical field (Figure 1, G).
Place the mouse in a cage softly heated laterally by an IR lamp for recovery after surgery. Make sure the lamp is not too close or too far from the cage (Figure 1, H).
Post-surgery care
Once awake and active, place the mouse into an ABSL-2 facility.
To overcome the possible post-operative pain, administer Temgésic (maximum 0,05 mg/kg) subcutaneously to the mice every 12h for 24h after surgery.
Closely watch the animals every day for at least one week to ensure their well-being. According to humane endpoints, mice reaching a score of non well-being or losing more than 20% of their body weight must be euthanized.

