BIDMC TMC - SOP for collection of peripheral lymphatic vessels
Nikolaos Kalavros, Ioannis Vlachos, Dhruv Singhal
Abstract
BIDMC Lymphatic TMC SOP for peripheral lymphatic vessel collection
Steps
Standard Operating Procedure Overview
Standard Operating Procedure to outline the process for obtaining, processing, and storing peripheral lymphatic vessel samples collected for the HuBMAP Lymphatic TMC.
The vessels are collected through a permanent removal of right axillary lymphatic vessel during lymphedema surgery from patients with malignant neoplasm of right female breast.
Donor Criteria
Patients with age >18 years from any race (White, Black, Hispanic, and Asian) are included.
Patients are excluded if they have one of the following characteristic:
- Have a known underlying lymphatic disease.
- Suffer from a chronic inflammatory condition (e.g. RA).
- Have ahistory of filarial infection.
- Have a history of allergy to lymphazurin blue.
- Are actively breastfeeding.
- Have a history of sensitivity to triphenylmethane.
- Have a history of systemic therapeutic treatment (e.g. chemotherapy).
- Have a history of pathology at anticipated site of lymphatic vessel harvest.
Donor metadata
The following information is recorded per study participant: Age, Sex, BMI, Hospitalization Time, Comorbidities, and Clinical history from the OMR.
Consenting
Following confirmation of the above inclusion and exclusion criteria by a study coordinator, the patient is informed about the study and provides informed consent according to the BIDMC IRB criteria.
Donors accepting to participate into the study are assigned an ascending 3-digit number (beginning with 001)
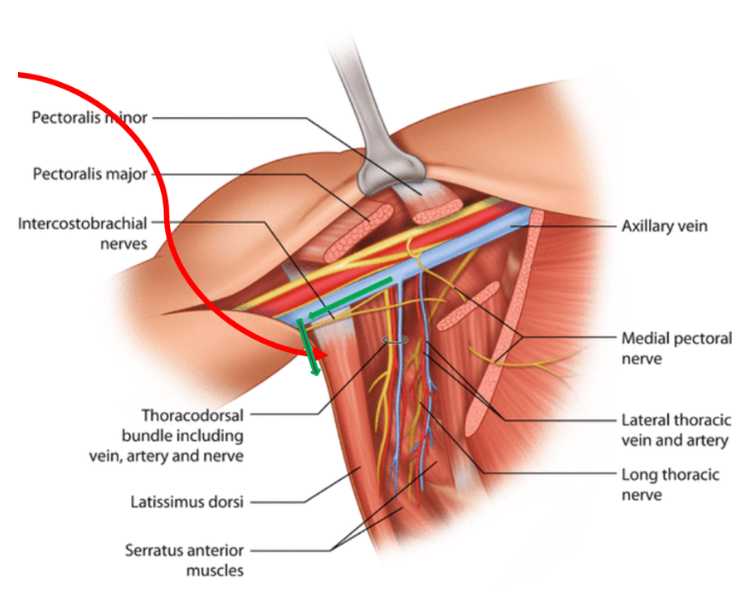
Collection of Peripheral Lymphatic Vessel
During the planned radial forearm free flap, 3 injections of 0.1cc of ICGare injected into the fascia 2cm apart proximal to the radial styloid.
The sites are massaged for 1 minute.
Identified lymphatic vessels are harvested (2x5mm, or longer) with microsurgical scissors and microclips applied to free lymphatic ends.
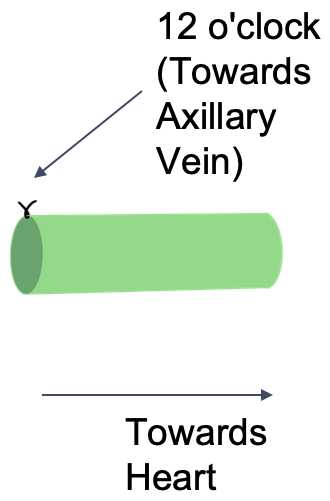
A 9-0 suture (peri-adventitial) is placed at the 12 o’clock position (i.e. the side of the vessel facing the axillary vein) – on the side farthest from the heart, to mark orientation.
The distance from thevessel to the edge of the axillary vein is recorded.
The peri-lymphatic adipose tissue of the lymphatic vessel is removed as closely to the vessel as possible.
Sample Processing
Prepare an isopentane and liquid nitrogen bath.
In a petri dish, carefully coat fresh tissue sample with room temperature OCT. Using a spatula, place the OCT-coated tissue into an appropriately sized cryomold.
Cover the tissue completely with OCT. Label the cryomold to mark the orientation of the tissue.
Using forceps, lower the embedded tissue into the isopentane without fully submerging.
Keep cryomold in contact with isopentane until the OCT has solidified and turned white. Once frozen, place the cryomold on powdered dry ice.
Store frozen embedded tissue in a sealed container at -80C or liquid nitrogen for long-term storage.

