Automated Protein Quantification with the Biomek-FX Liquid Handler System
Yan Chen, Nurgul Kaplan Lease, Jennifer Gin, Tad Ogorzalek, Christopher J Petzold
Abstract
This protocol details steps to perform the protein quantification (Lowry-based) assay by using a Biomek FX liquid handler system. It is optimized to assay a full 96-well plate of protein samples in duplicate with a separate (control) plate for BSA standards. You will need a plate reader to measure the samples and standards.
This protocol works best as part of a full proteomic sample preparation workflow with:
Automated Chloroform-Methanol Protein Extraction on the Biomek-FX Liquid Handler System
and
Automated Protein Normalization and Tryptic Digestion on a Biomek-FX Liquid Handler System
Before start
Prepare BSA Standards Plate (1st 4 rows from A to D):
-
Add 40 uL of H2O into wells A1 to D1.
-
Add 40 uL of BSA Standards 1 (125 ug/mL) to 7 (2000 ug/mL) into columns 2 to 8. For this protocol you will need:
-
Beckman-Coulter Biomek FX liquid handler system with a 96-pod head.
-
Upload the attached method file and modify it to fit your deck and system configuration. Modular Protein Quantitation method.bmf
Steps
Deck Setup
Open Biomek Software that controls Biomek-FX liquid handler system. Under "File" drop down click "Open" to select the automation method "Modular Protein Quantitation method"
Click on "Instrument Setup" under the "Setup" group node to get visual instruction of how to set up the deck.
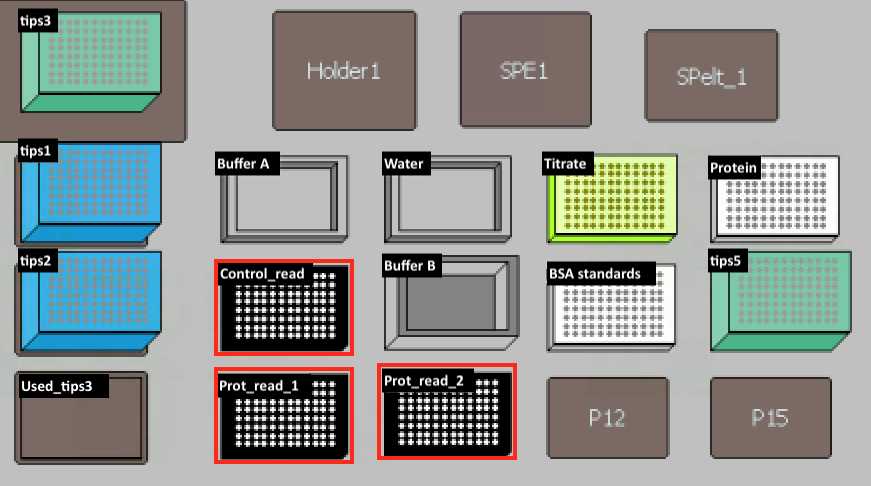
Set up the deck (refer to the deck setup picture below):
| A | B | C |
|---|---|---|
| Deck Label | Labware | Reagent |
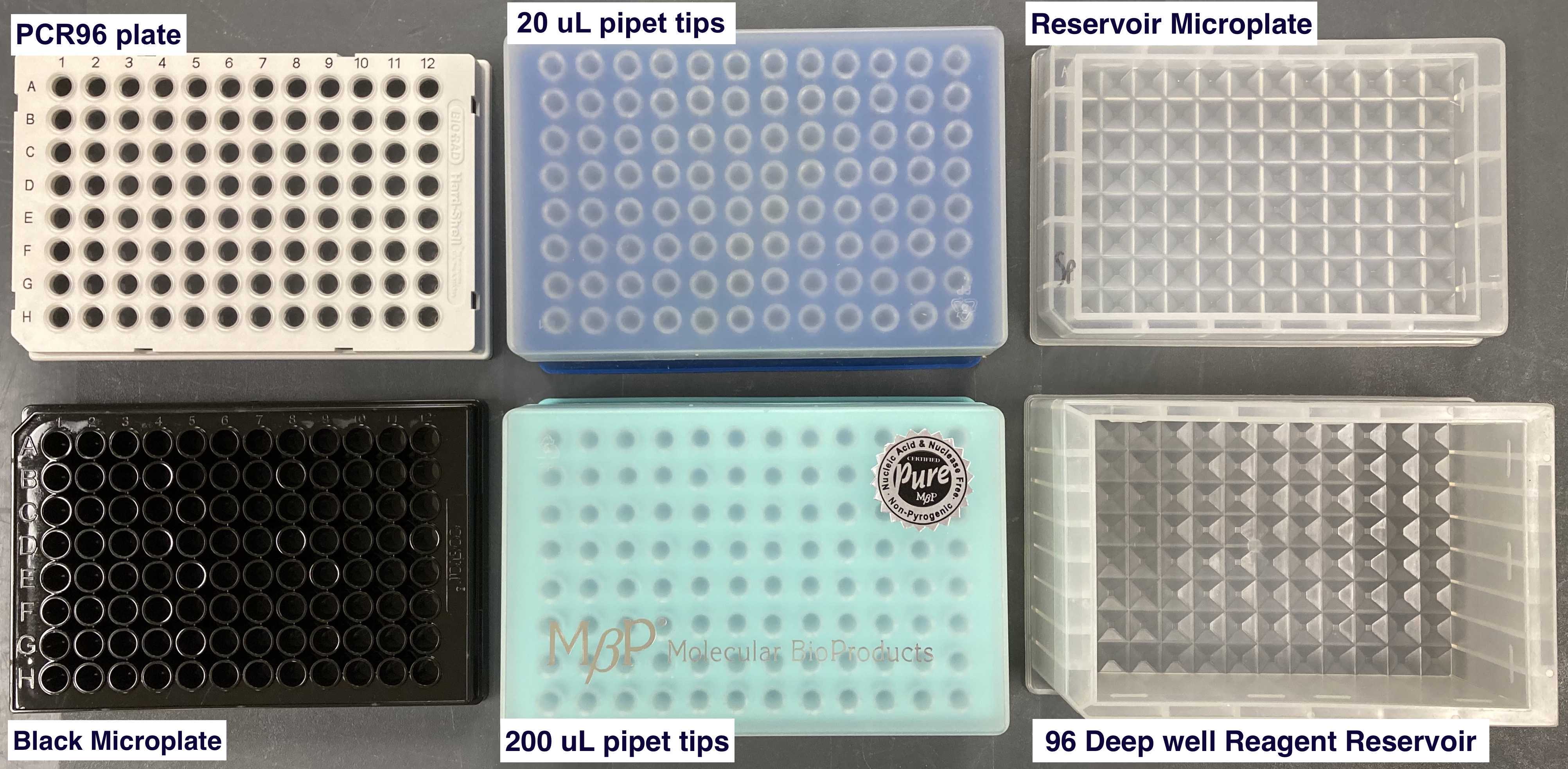
| protein | PCR96 plate (BIO-RAD, Cat.#HSP9601) | unknown amount of protein to quantify |
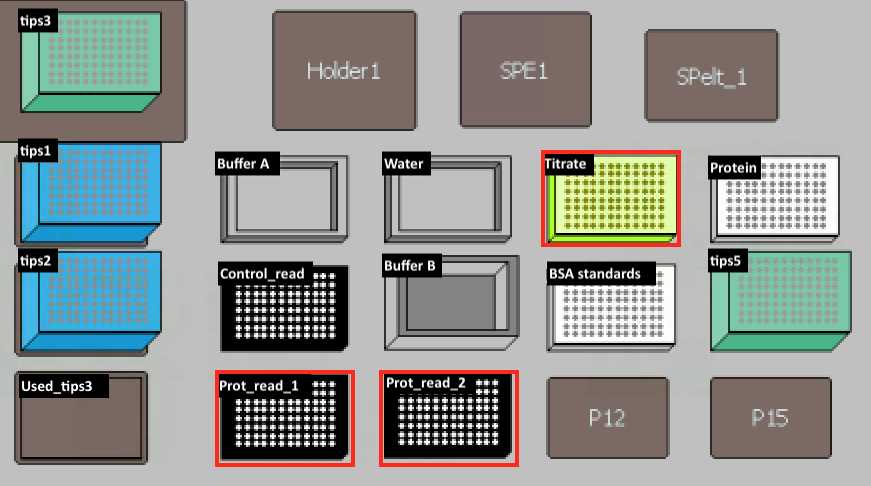
| titrate | PCR96 plate (BIO-RAD, Cat.#HSP9601) | |
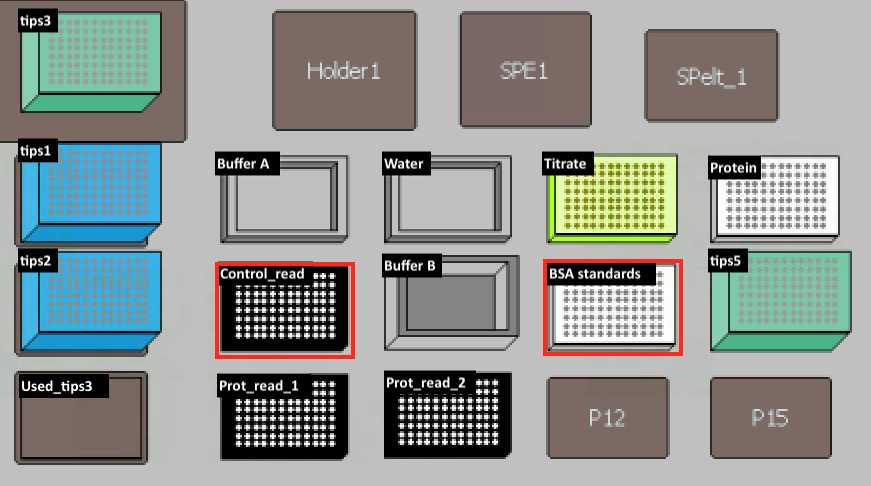
| BSA standards | PCR96 plate | BSA Standards (Thermo Fisher, Cat.#23208) |
| tips1,2 | 20 µl pipet tips (Molecular Bioproducts BioRobotix, Cat.#918-262 ) | |
| tips 3,5 | 200 uL pipet tips (Molecular Bioproducts BioRobotix, Cat.#919-262 ) | |
| control read, prot read 1, prot read 2 | Black Microplate (Fisher Scientific, Cat.#07-200-567) | |
| Buffer A | Reservoir Microplate (Agilent, Cat.#201254-100) | DC Protein Assay Reagent A (Bio-rad Laboratories, Cat.#500-0113) |
| water | Reservoir Microplate (Agilent, Cat.#201254-100) | LC-MS grade Water (VWR Scientific, Cat.#BJLC365-2.5) |
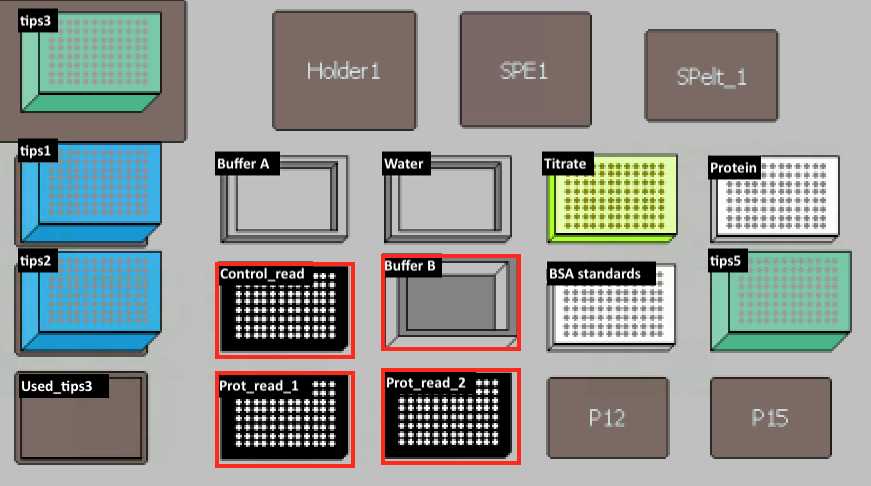
| Buffer B | 96 Deep Well Reagent Reservoir (VWR, Cat.#101100-962) | DC Protein Assay Reagent B (Bio-rad Laboratories, Cat.#500-0114) |
Materials for Deck setup
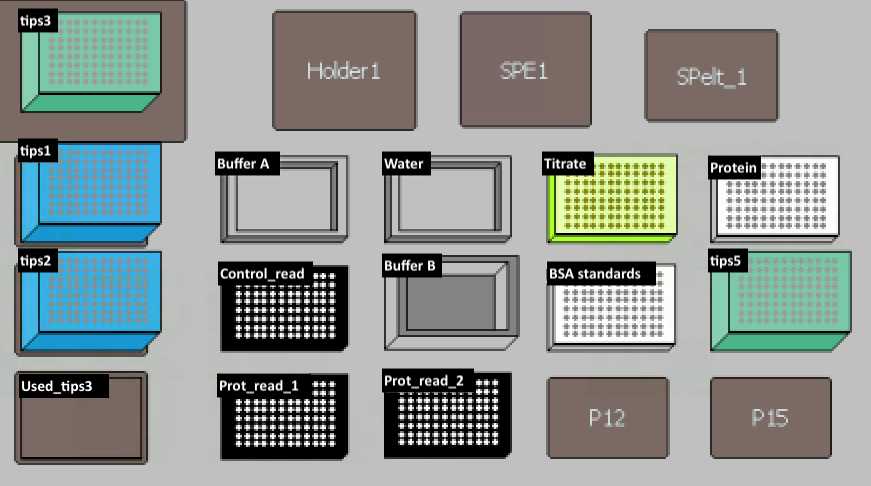
MANUAL STEP: Use a multichannel pipette to mix protein samples right before starting.
Click the "Run" button (green arrow) to start.
DC protein assay
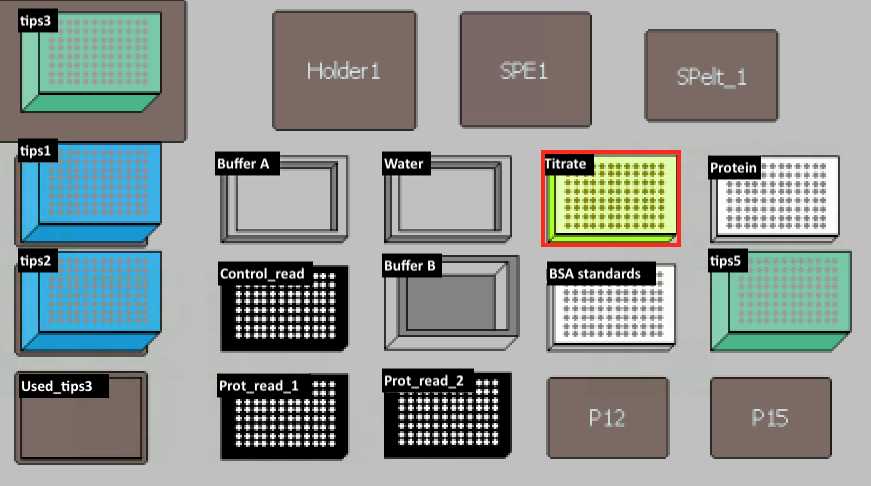
Transfer 20 µl of H2O into Titrate Plate. Then transfer 5 µl from protein plate to titrate plate and mix on deck.
Method will pause until user resume it again. Set a timer for 5 minutes.
After 5 min., click OK to resume method
Spectrophotometer reading for protein Quantification
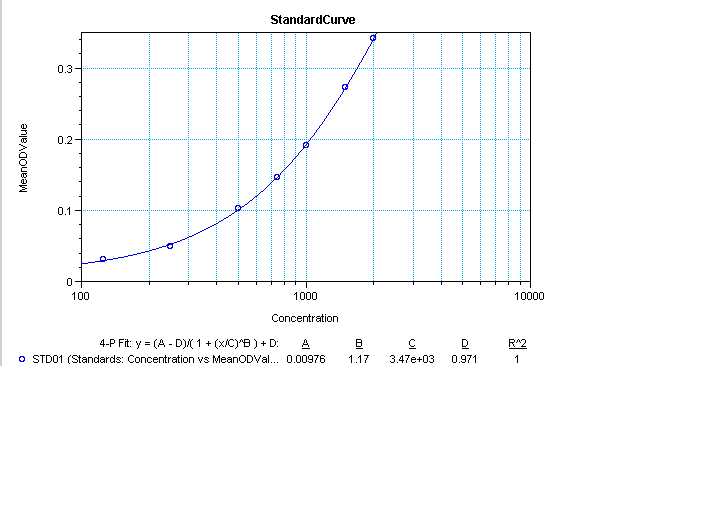
Transfer plates to the microplate reader (MD Spectramax 250) to read absorbance at 750 nm and calculate protein concentration.
Read "prot read 1" and "prot read 2" plates.
| A | B |
|---|---|
| Sample | Concentration |
| Un88 | 585.249 |
| Un89 | 785.257 |
| Un90 | 670.135 |
| Un91 | 718.864 |
| Un92 | 868.962 |
| Un93 | 679.907 |
| Un94 | 743.064 |
| Un95 | 994.173 |
| Un96 | 1115.072 |
Data concentrations example
Store protein plate at -20°C until ready for Automated Protein Normalization and Tryptic Digestion on a Biomek-NX Liquid Handler System