Automated Chloroform-Methanol Protein Extraction on the Biomek-FX Liquid Handler System
Yan Chen, Nurgul Kaplan Lease, Jennifer Gin, Tad Ogorzalek, Christopher J Petzold
Abstract
This protocol details steps to extract protein from Gram-negative bacterial or fungal cells (that have been pretreated with zymolyase) in quantitative proteomic workflows by using a Biomek FX liquid handler system. It is a semi-automated protocol that includes several 'pause' steps for centrifugation steps that are conducted manually "off-deck".
This protocol works best as part of an automated proteomic sample preparation workflow with:
Automated Protein Quantitation with the Biomek-FX liquid handler system
and
Automated Protein Normalization and Tryptic Digestion on a Biomek-NX Liquid Handler System
Before start
For this protocol you will need:
-
a Beckman-Coulter Biomek FX liquid handler system with a 96-pod head
-
Upload the attached method file and modify it to fit your deck and system configuration
Modular Protein Extraction method.bmf
- an Eppendorf 5810R centrifuge with S-4-104 rotor or similar centrifuge
Steps
Deck Setup
Open Biomek Software that controls Biomek-FX liquid handler system. Under "File" drop down click "Open" to select the automation method "Modular Protein Extraction method"
Click on "Instrument Setup" under the "Setup" group node to get visual instruction of how to set up the deck.
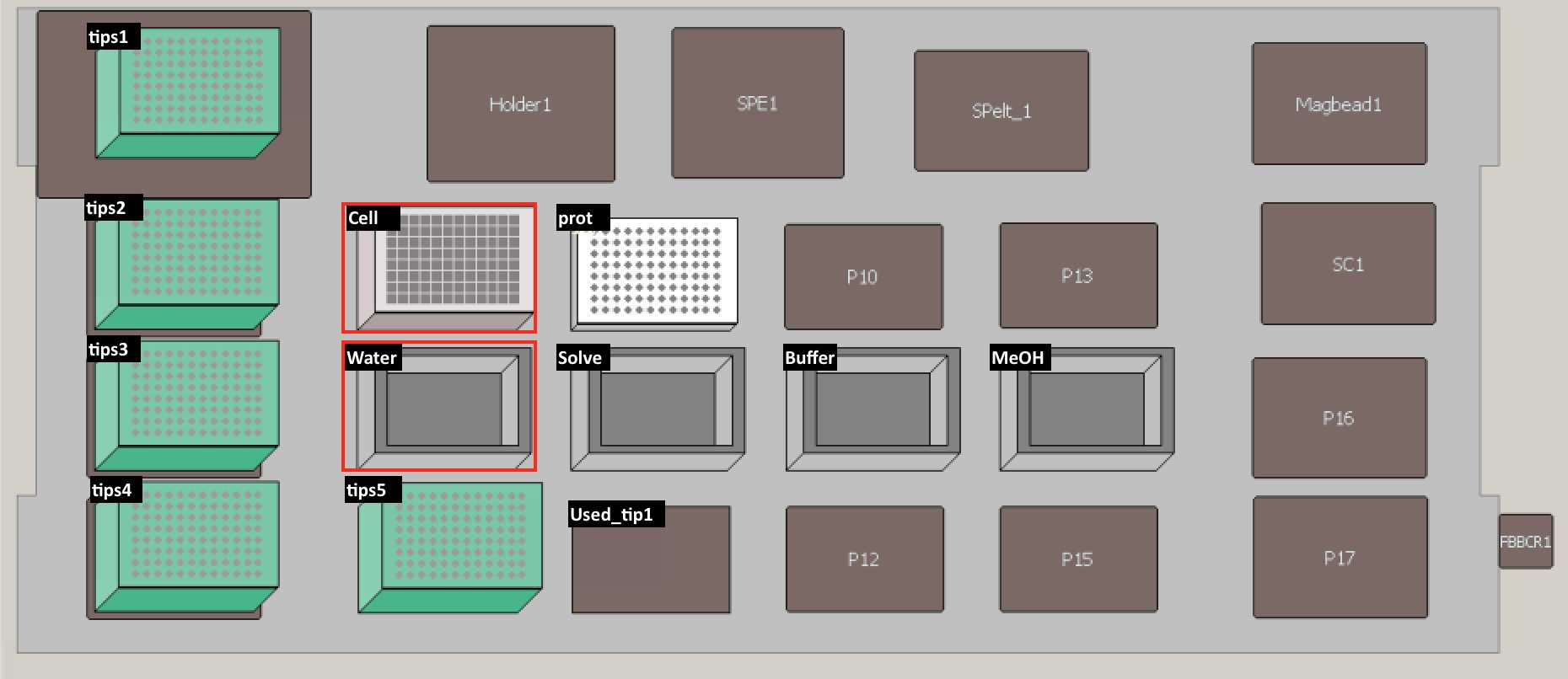
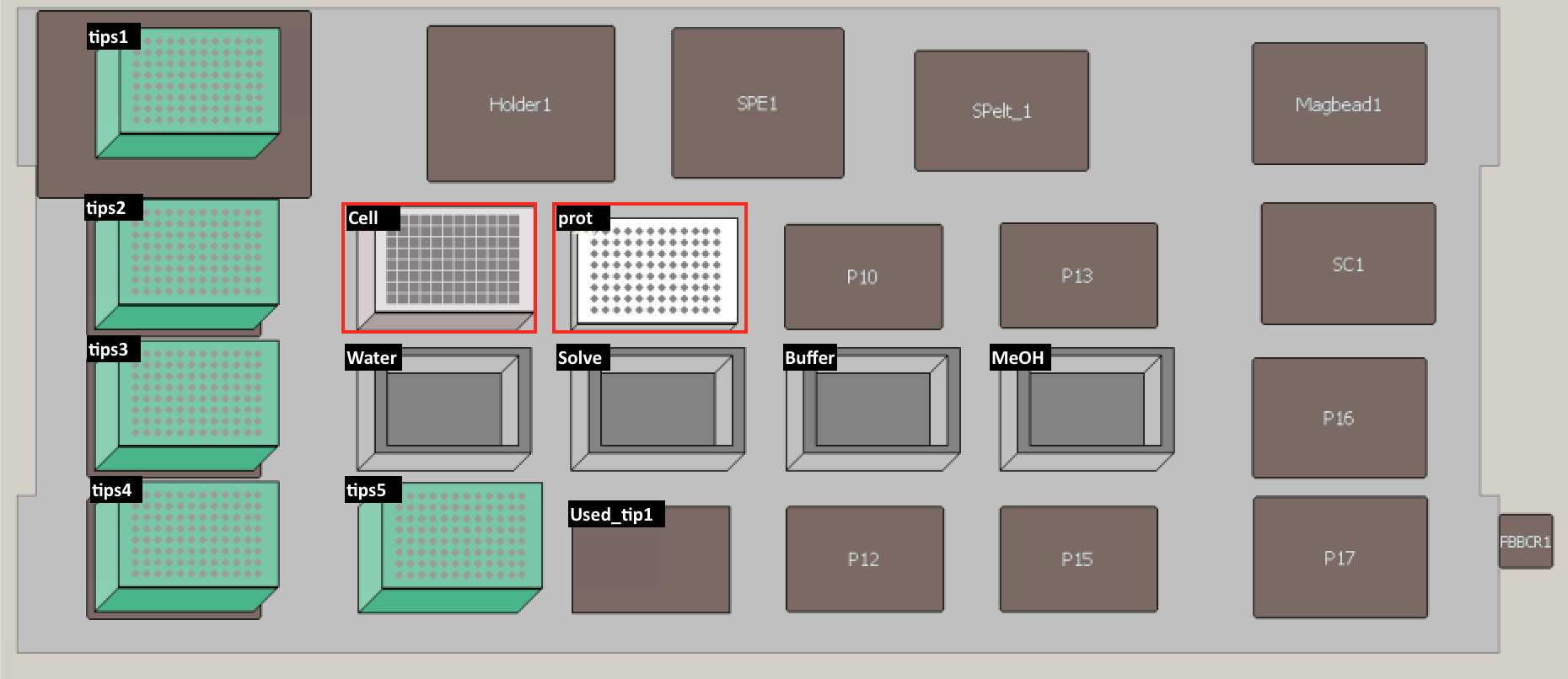
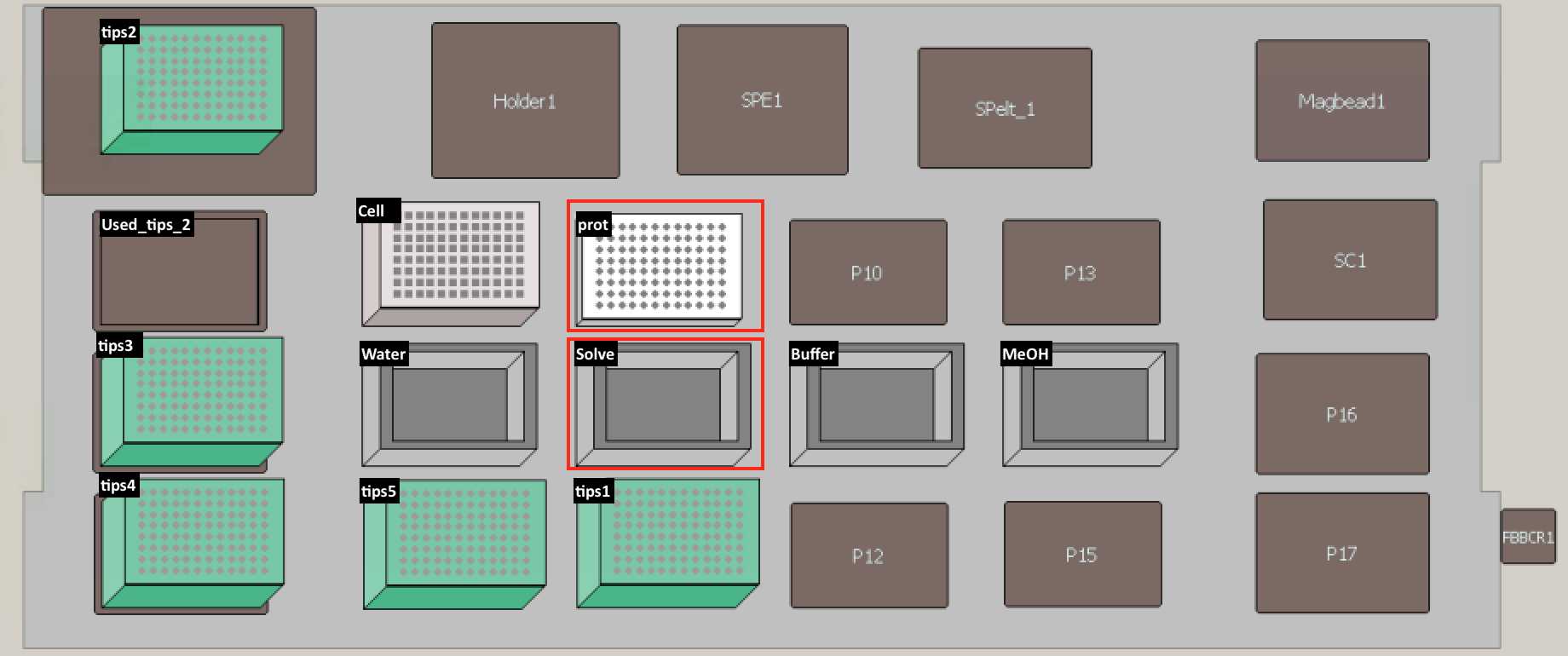
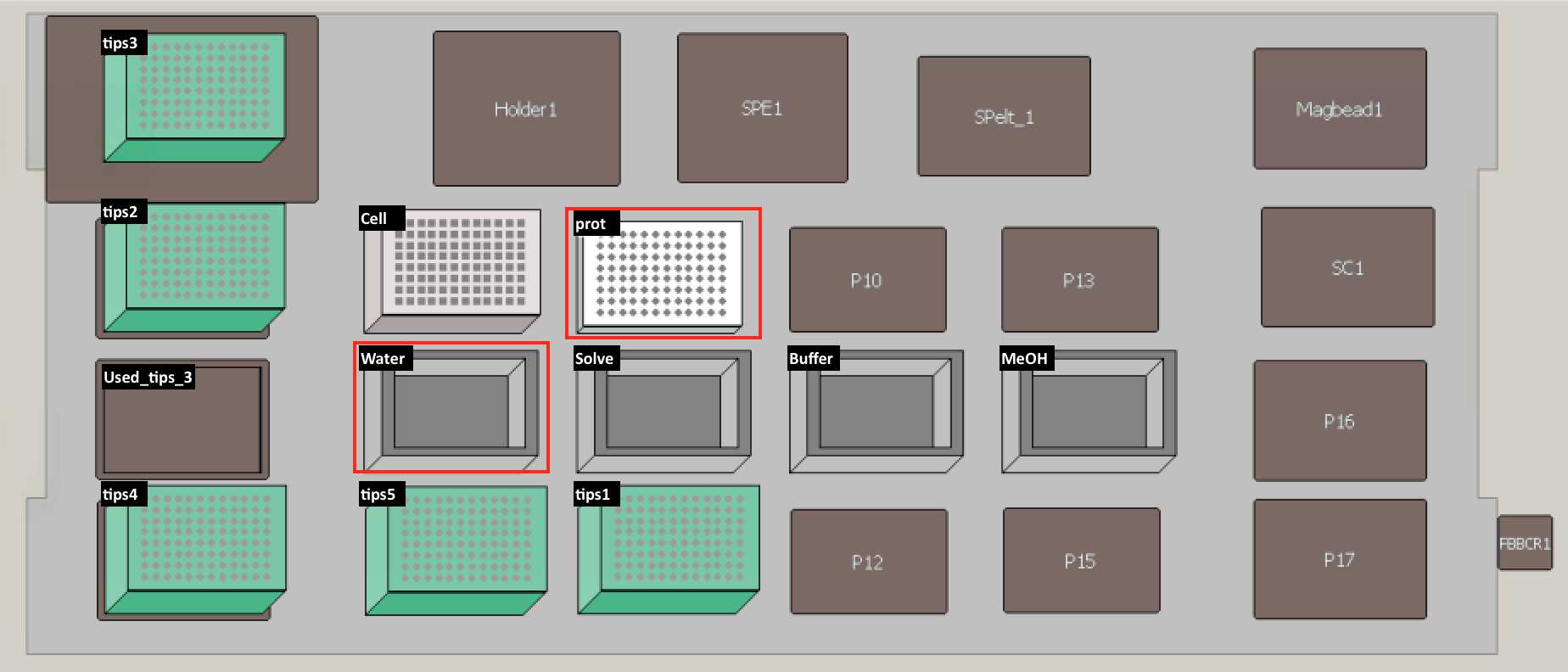
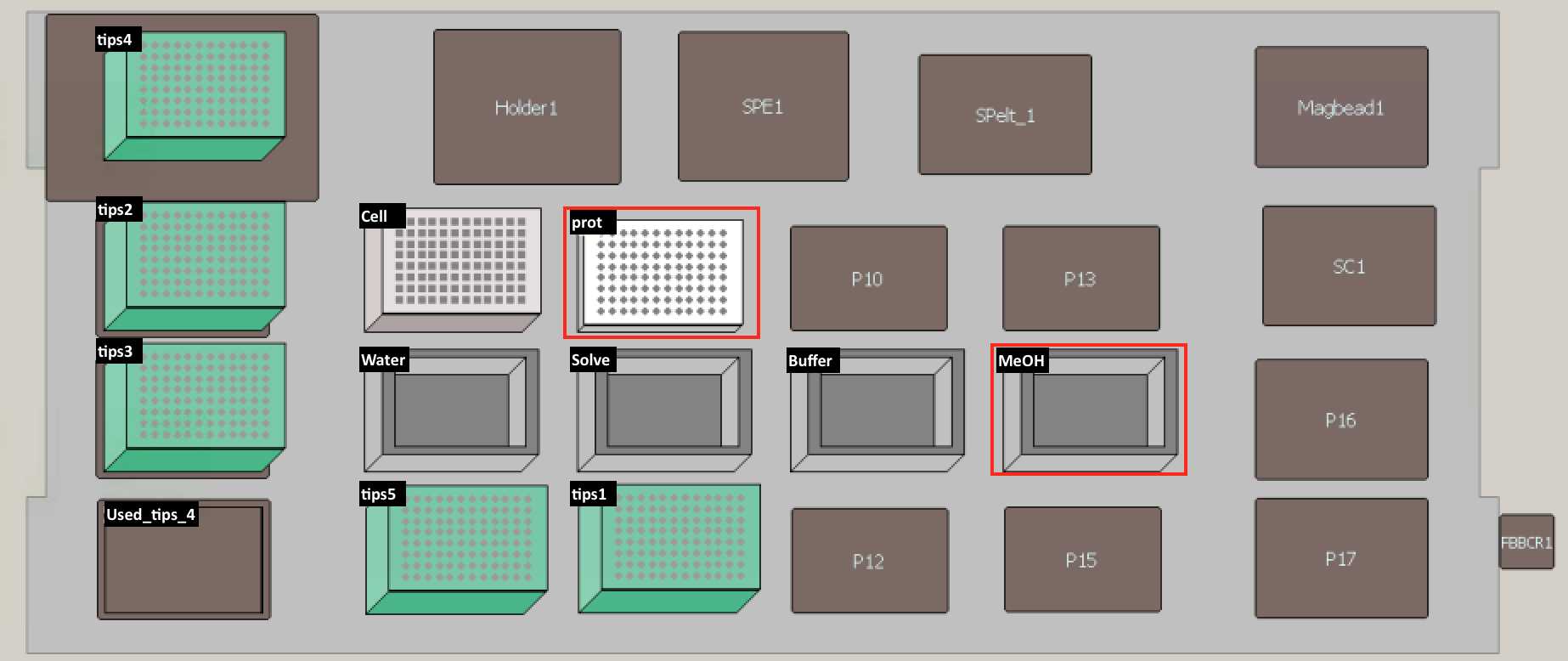
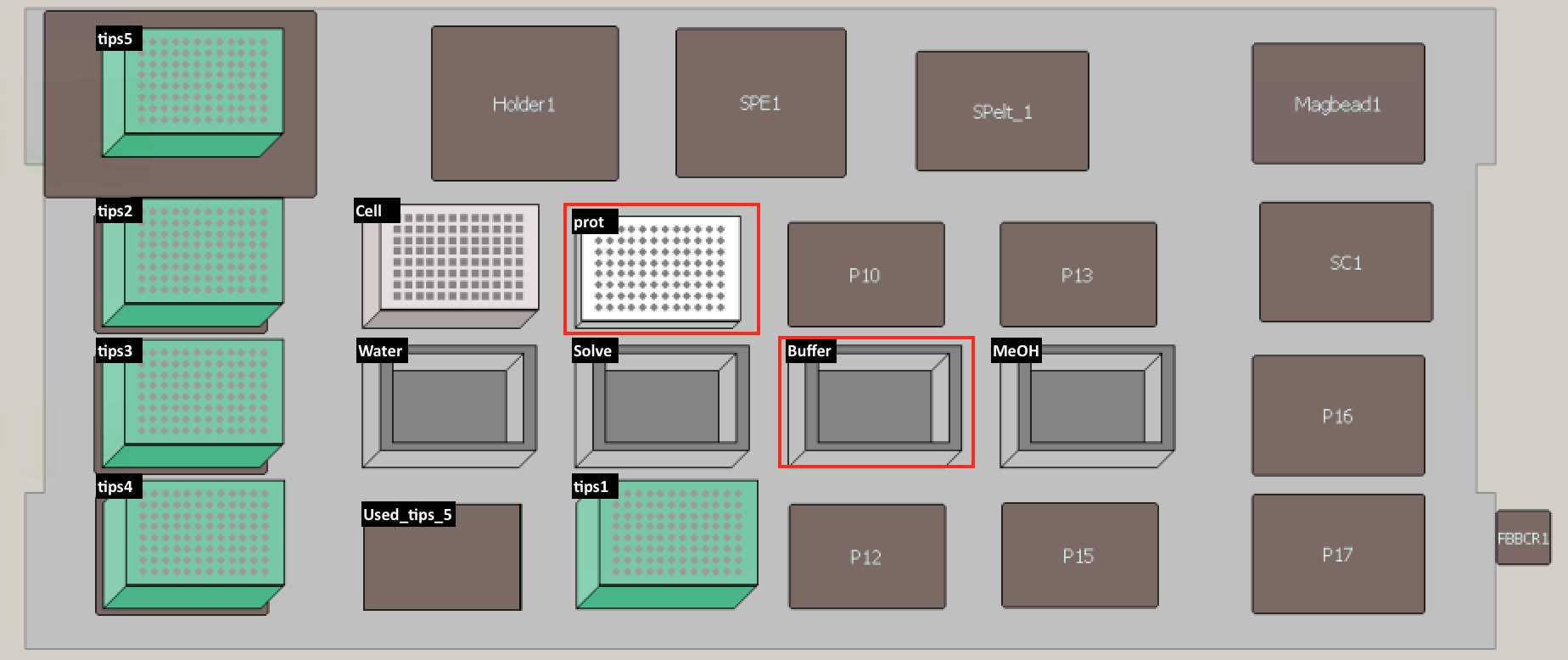
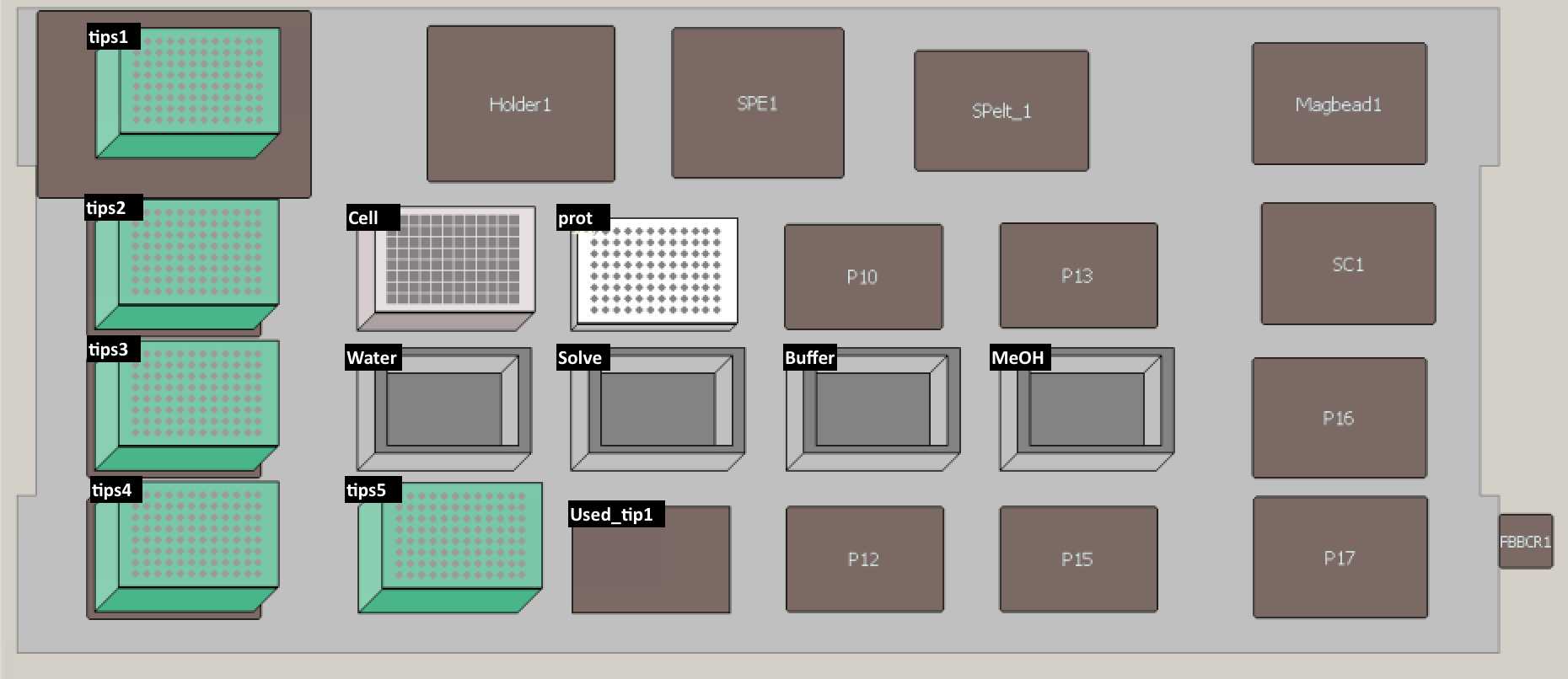
Set up the deck (refer to the deck setup picture below):
| A | B | C |
|---|---|---|
| Deck Label | Labware | Reagent |
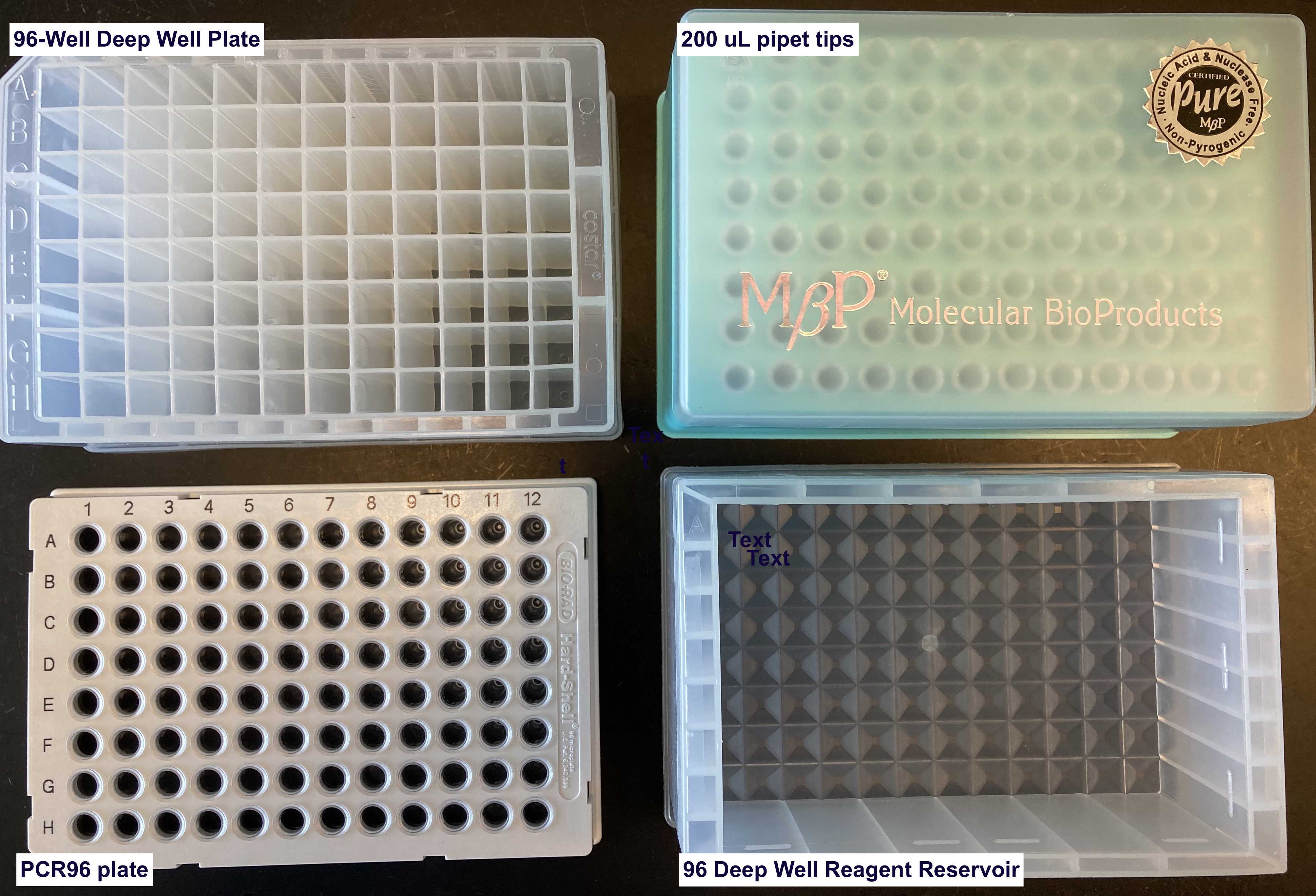
| cells | 96-Well Deep Well Plate (Sigma Aldrich, Cat.#CLS3961-100EA) | 4 OD units of cells |
| prot | PCR96 plate (BIO-RAD, Cat.#HSP9601) | |
| tips1-5 | 200 uL pipet tips (Molecular Bioproducts BioRobotix, Cat.#919-262 ) | |
| water | 96 Deep Well Reagent Reservoir (VWR, Cat.#101100-962) | LC-MS grade Water (VWR Scientific, Cat.#BJLC365-2.5) |
| solvent | 96 Deep Well Reagent Reservoir (VWR, Cat.#101100-962) | 4:1 Methanol:Chloroform LC-MS grade Methanol (VWR Scientific, Cat.#BJLC230-2.5), Chloroform (Sigma-Aldrich, Cat.#34854) |
| buffer | 96 Deep Well Reagent Reservoir (VWR, Cat.#101100-962) | 100 milimolar (mM) Ammonium Bicarbonate in 20% Methanol |
| MeOH | 96 Deep Well Reagent Reservoir (VWR, Cat.#101100-962) | LC-MS grade Methanol (VWR Scientific, Cat.#BJLC230-2.5) |
Materials for Deck setupNote: 1 OD unit = 1 ml of cell culture at OD600 measurement of 1.0
Click the "Run" button (green arrow) to start.
Protein Extraction
The PAUSE step prompts you to centrifuge your plate.
Centrifuge 2000x g,25°C .
Put plate back on deck space (refer to deck setup picture above) -- PCR96 plate "prot." Swirl and pour solvent (4:1, Methanol:Chloroform) into reservoir.
Remove supernatant by transferring 87 µl from PCR96 plate back to cell plate.
The PAUSE step prompts you to centrifuge your plate.
Centrifuge 4000rpm,25°C
Put plate back on deck space (refer to deck setup picture above) -- PCR96 plate "prot."
Remove supernatant by transferring 170 µl top layer from PCR96 plate to cell plate.
The PAUSE step prompts you to centrifuge your plate.
Centrifuge 4000rpm,25°C
Put plate back on deck space (refer to deck setup picture above) -- PCR96 plate "prot."
Remove supernatant.
Store at -20°C until ready for Automated Protein Quantitation with the Biomek-FX liquid handler system