812.1 Lung FFPE OMAP Multiplexed Immunofluorescence Phenocycler-Fusion® Antibody Validation Protocol
Gloria S Pryhuber, Heidie Huyck, gail.deutsch, Jeffrey Purkerson
Abstract
This protocol describes validation of a 34-antibody panel used for multiplexed immunofluorescent (MxF) staining and imaging of FFPE lung tissue sections utilizing the Phenocycler-Fusion® platform (Akoya Biosciences).
The validation strategy involves staining two serial lung sections, one with cocktail of custom barcode-conjugated antibodies plus antibody-barcoded conjugates sourced from Akoya Biosciences and the second with the respective unconjugated, carrier-free control antibodies and omitting the Akoya antibodies.
In addition, a tonsil section was exposed to the all-conjugated antibody cocktail to confirm specificity of antibodies directed against lung specific markers, and specificity of pan-epithelial, endothelial, megakaryocyte, and immune cell markers in 2 different tissues (e.g. lung, tonsil).
The protocol includes sections describing 1) Tissue sections, 2) Labeling with barcode-conjugated or unconjugated Ab, 3) Reporter Plate and Experimental Design, 4) Multiplexed Imaging and Image Upload to OMERO for review, and 5) Review criteria. Detailed descriptions of staining and imaging procedures can be found in a companion protocol dx.doi.org/10.17504/protocols.io.6qpvr38dpvmk/v2.
Before start
Review HubMAP guidelines for Ab validation in the attached SOP for OMAP construction
Attachments
Steps
Tissue Sections
Lung serial sections, and a tonsil section were prepared in FFPE as described in dx.doi.org/10.17504/protocols.io.kxygxejwdv8j/v2
Labeling of Tissue Sections with barcode conjugated Ab or unconjugated Ab
One of two serial healthy Lung sections (D016-RLL-11B2-6), a known diseased Lung section (D115-RLL-11A2-19) and a Tonsil Section (Tonsil-11-5) was labeled with a current panel (Table 1) of barcode antibodies as described in dx.doi.org/10.17504/protocols.io.6qpvr38dpvmk/v2. The other lung serial section (D016-RLL-11B2-5) was labeled with a panel of carrier free, unconjugated antibodies used for custom conjugations from step 18.1 in the above protocol and are listed in Table 1.
Table 1. Antibodies used for custom conjugations.
| A | B | C | D | E |
|---|---|---|---|---|
| TPSAB1-BX041 | TPSAB1 | 1:1000 | Abcam | ab2378 |
| SFTPC-BX020 | SFTPC | 1:500 | Invitrogen | PA5-71842 |
| ß-III-Tubulin-BX055 | ß-III-Tubulin | 1:400 | R&D Systems | MAB1195 |
| SCGB1A1-BX043 | SCGB1A1 | 1:400 | R&D Systems | MAB4218 |
| SCGB3A2-BX002 | SCGB3A2 | 1:400 | Abcam | ab240255 |
| CXCL4-BX004 | CXCL4 | 1:200 | Peprotech | 500-P05 |
| PROX1-BX050 | PROX1 | 1:200 | R&D Systems | AF2727 |
| LYVE1-BX025 | LYVE1 | 1:100 | R&D Systems | AF2089 |
| RAGE-BX028 | RAGE | 1:100 | Abcam | ab228861 |
| CD298-BX005 | CD298 | 1:100 | Abcam | ab167390 |
| MUC5AC-BX040 | MUC5AC | 1:100 | Abcam | ab212636 |
| SCEL-BX052 | SCEL | 1:100 | Abcepta | AP11564C |
| TP63-BX006 | TP63 | 1:100 | Abcam | ab214790 |
| COL1A1-BX054 | COL1A1 | 1:100 | Abcam | ab88147 |
| EDNRB-BX027 | EDNRB | 1:50 | R&D Systems | MAB4496 |
| CD1c-BX016 | CD1c | 1:50 | Novus | ab156708 |
For antibodies containing sodium azide (0.05-0.1%) or trehalose (5%), buffer exchange was performed utilizing Zeba Spin Desalting columns 7K MWCO (89890, 2ml, Thermoscience) equilibrated in PBS in accordance with the manufacturer's recommendations.
Table 2. Antibodies commercially conjugated (Akoya Biosciences).
| A | B | C | D |
|---|---|---|---|
| PanCK-BX019 | 1:200 | Akoya | 4450020 |
| COLIV-BX042 | 1:200 | Akoya | 4450122 |
| Keratin5-BX101 | 1:200 | Akoya | 4450090 |
| ECAD-BX014 | 1:200 | Akoya | 4250021 |
| CD45-BX021 | 1:200 | Akoya | 4550121 |
| Ki67-BX047 | 1:200 | Akoya | 4250019 |
| CD68-BX015 | 1:200 | Akoya | 4550113 |
| CD14-BX037* | 1:200 | Akoya | 4450047 |
| CD163-BX069 | 1:200 | Akoya | 4250079 |
| CD4-BX003 | 1:200 | Akoya | 4550112 |
| CD8-BX026 | 1:200 | Akoya | 4250012 |
| CD3e-BX045 | 1:200 | Akoya | 4550119 |
| FOXP3-BX031 | 1:200 | Akoya | 4550071 |
| CD20-BX007 | 1:200 | Akoya | 4450018 |
| HLADR-BX033 | 1:200 | Akoya | 4550118 |
| CD11c-BX024 | 1:200 | Akoya | 4550114 |
| MPO-BX098 | 1:200 | Akoya | 4250083 |
| CD31-BX001 | 1:200 | Akoya | 4450017 |
| SMA-BX013 | 1:200 | Akoya | 4450049 |
Note that unconjugated forms of barcode-conjugated antibodies purchased from Akoya Biosciences are not included for validation. The majority of Akoya-sourced antibody clones have previously been validated for Akoya multiplexed immunofluorescence assessment of FFPE tissue. The antibody dilutions correspond to working dilutions for the respective barcode conjugated Ab. *CD14-BX037 was omitted from the OMAP-34 validation as the Ab was temporarily unavailable. However, this Ab has been validated on FFPE tissue by others and in prior experiments.
Reporter Plate and Experiment Design
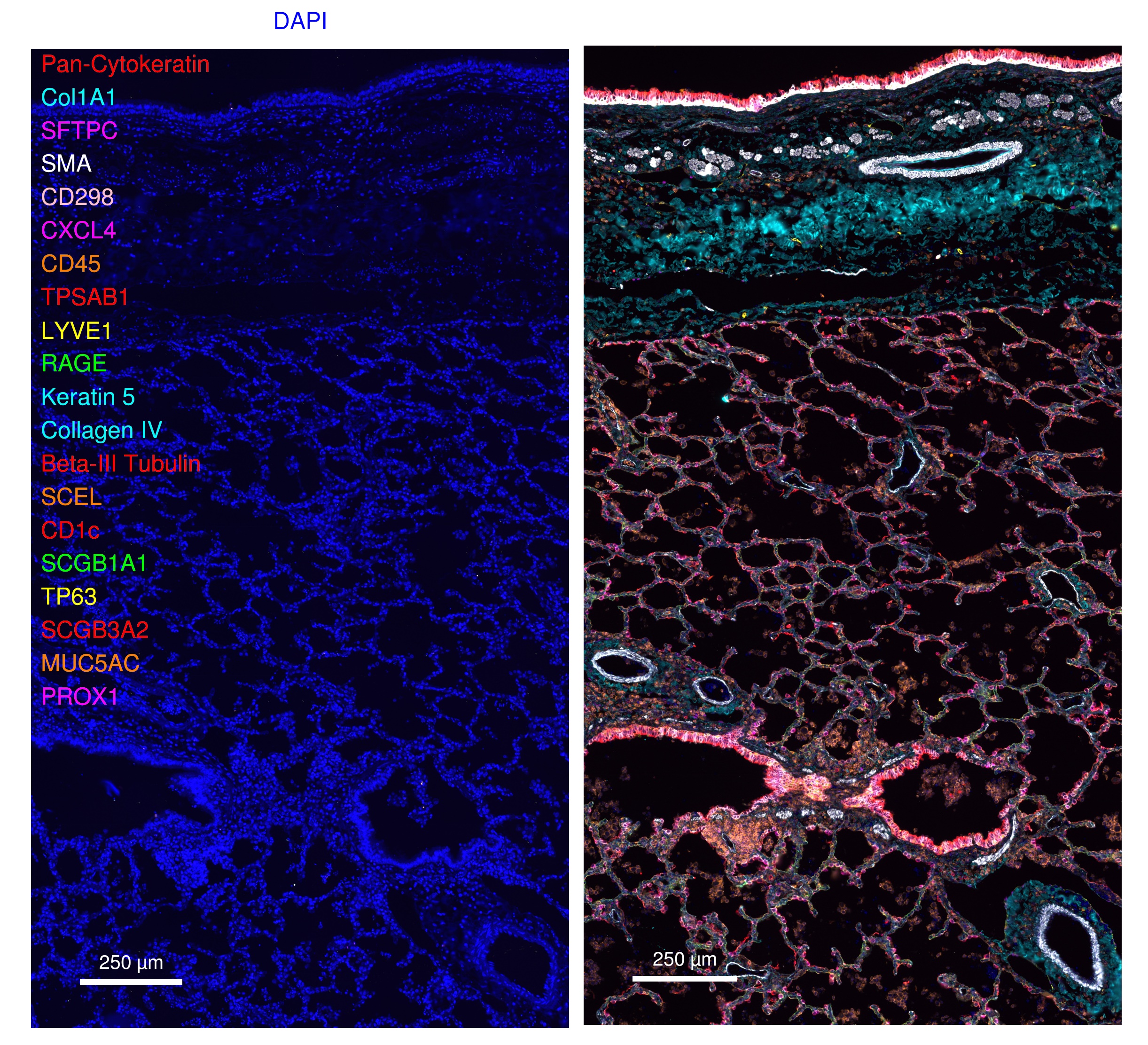
Reporter plate design and Phenocycler-Fusion run protocols were developed using the PhenoCycler Experiment Designer Software (Akoya Biosciences) as described in dx.doi.org/10.17504/protocols.io.6qpvr38dpvmk/v2. The Reporter Plate design and exposures used for validation of the Lung FFPE OMAP-34 Panel is shown in Table 3. Note that all 3 slides included in the validation were exposed to the same reporter panel.
Table 3. Lung FFPE OMAP-34 Reporter Plate Design and exposures
Cycle #, reporters and exposure times
| A | B | C | D | E | F |
|---|---|---|---|---|---|
| Ki67-RX047 | 125 | CD68-RX015 | 25 | PanCK-RX019 | 150 |
| CD8-RX026 | 150 | CD4-RX003 | 75 | CD20-RX007 | 150 |
| Col1A1-RX054 | 400 | CD3e-RX045 | 50 | CD31-RX001 | 150 |
| SFTPC-RX020 | 100 | HLADR-RX033 | 50 | SMA-RX013 | 150 |
| CD298-RX005 | 100 | CD45-RX021 | 75 | CXCL4-RX004 | 150 |
| TPSAB1-RX041 | 10 | FOXP3-RX031 | 150 | LYVE1-RX025 | 400 |
| CD163-RX069 | 250 | CD11c-RX024 | 125 | RAGE-RX028 | 200 |
| MPO-RX098 | 25 | COlIV-RX042 | 150 | Keratin5-RX101 | 75 |
| ß-III-Tub-RX055 | 250 | CD1c-RX016 | 300 | SCEL-RX052 | 400 |
| ECAD-RX014 | 200 | TP63-RX006 | 150 | SCGB1A1-BX043 | 125 |
| SCGB3A2-RX002 | 75 | ENDRB-RX027 | 250 | MUC5AC-RX040 | 150 |
| PROX1-RX050 | 150 | None | 150 | None | 150 |
DAPI exposure 3 milliseconds (ms), CD14-RX037 omitted due unavailability of Ab-barcode.
Multiplexed Imaging and Upload to OMERO for review
Tonsil and Lung Sections were imaged as described in dx.doi.org/10.17504/protocols.io.6qpvr38dpvmk/v2.
OMAP-34 Validation consisted of the following slide runs.
-
Tonsil 11-5 (OMAP-34 Antibody Panel)
-
D016-RLL-11B2-5 (Unconjugated Ab panel)
-
D016-RLL-11B2-6 (OMAP-34 Antibody Panel)
-
D115-RLL-11A2-19 (OMAP-34)
The resulting .qptiff images are converted to ome.tiffs utilizing command line: C:>bfconvert -no-sas -series 0 -compression LZW -pyramid-resolutions 5 -pyramid-scale 2 INPUT.qptiff OUTPUT_ome.tiff.
Utilizing Omero importer, ome.tiff files are uploaded to the URMC Omero-CODEX folder and the respective validation subfolder (i.e. OMAP34validation) for review.
Review Criteria
Validation of marker signals is reviewed by a 3-person panel that includes the protocol author (MxIF Research Scientist), Dr. Gloria Pryhuber (Principal investigator), and Dr. Gail Deutsch (Board Certified Pathologist, Pulmonary specialist).
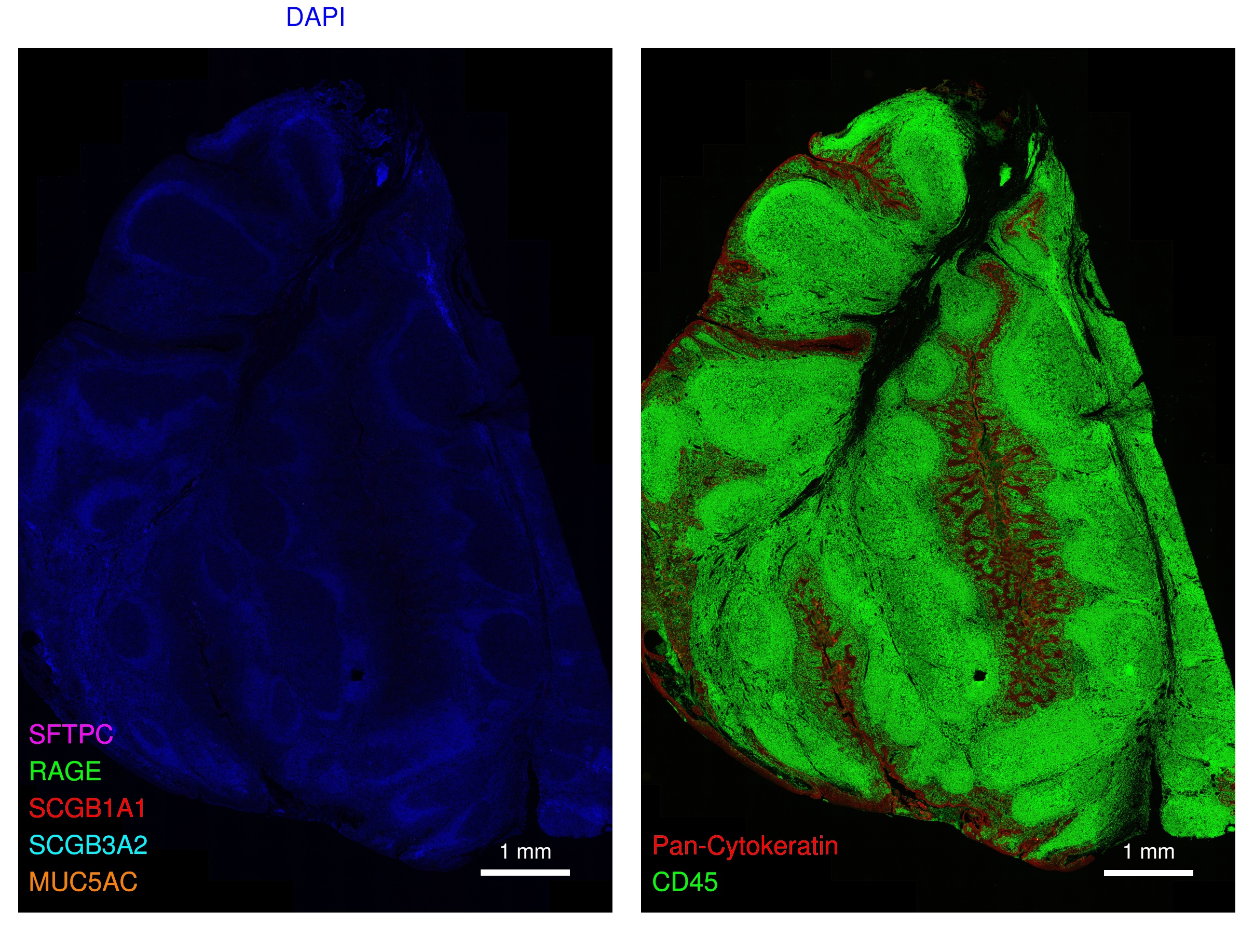
Lung and tonsil sections are reviewed for marker fluorescence signal localization to appropriate anatomical structures (e.g. airway, vasculature) and cell types within anatomical structures (e.g. alveolar epithelia, vascular endothelium, immune cell clusters), colocalization with appropriate pan-markers (e.g. CD4 with CD3/CD45, CD11c with HLADR/CD45; SCEL with RAGE and pan-cytokeratin (pan-CK), etc.).
Slides labeled with unconjugated Ab are assessed for the contribution, if any, of nonspecific reporter binding to generate fluorescent signal.
The tonsil section(s) was reviewed for any nonspecific interactions of respective Ab-barcode conjugates expected to uniquely target lung specific markers (e.g. RAGE, SFTPC, SCGB1A1, SCGB3A2, MUC5AC).